Research Journal for Veterinary Practitioners
Case Report
Correction and Management of Vaginal Prolapse in A Cow by Buhner’s Technique
Tanjila Hasan1, Azizunnesa1*, Md. Anowar Parvez 1, Pranab Paul1, Sharmin Akter1, Md. Omer Faruk2, Delower Hossain3
1Department of Medicine and Surgery, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong-4225, Bangladesh; 2Upazilla Livestock Officer (ULO), Department of Livestock Services (DLS), Farmgate, Dhake-1215, Bangladesh; 3MS Student, Department of Medicine and Surgery, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong-4225, Bangladesh.
Abstract | Vaginal prolapse is a common obstetrical disorder for dairy cows throughout the country during 3rd trimester of gestation. But there is no available published report on it in Bangladesh. A six years old HF cross was admitted to Teaching Veterinary Hospital (TVH) in Chittagong Veterinary and Animal Sciences University (CVASU) with a history of 7 months pregnancy and hanging mass outside the genital opening. By physical examination, it was confirmed as vaginal prolapse. After that, blood sample was collected to know different parameters of serum e.g. calcium, phosphorus, magnesium etc. The prolapsed mass was corrected manually and Buhner’s suture was applied parallel to vulva apart from vagina beneath the skin to keep it in position. The cow was followed for next 2 months. There found no evidence of recurrence and then it delivered a healthy female calf successfully without any complication. The aim of the report is to show successful correction of vaginal prolapse with its management by quick diagnosis and immediate action.
Keywords | Vaginal prolapse, Correction, Management, Buhner’s technique
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | February 06, 2017; Accepted | March 06, 2017; Published | March 15, 2017
*Correspondence | Azizunnesa, Dept. of Medicine and Surgery, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong-4225, Bangladesh; Email: msrekha04@yahoo.com
Citation | Hasan T, Azizunnesa, Parvez MA, Paul P, Akter S, Faruk MO, Hossain D (2017). Correction and management of vaginal prolapse in a cow by Buhner’s technique. Res. J. Vet. Pract. 5(1): 1-4.
DOI | http://dx.doi.org/10.17582/journal.rjvp/2017/5.1.1.4
ISSN | 2308-2798
Copyright © 2017 Azizunnesa. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Genital prolapses are mostly seen in ruminants specially cattle, buffalo, sheep, goat (Patra et al., 2014). It may be defined as coming out of one or more of the pelvic structures (bladder, uterus and vagina) from their normal anatomical position through the genital (vaginal) opening. Among all prolapse, uterine and vaginal prolapse are frequently observed in the reproductive tract of cow (Powell, 2007). Vaginal prolapse mostly happened in cross breed cattle before calving, usually in the last trimester of pregnancy (Roberts, 1971). But there was also evidence of post oestral vaginal prolapse in a non-pregnant heifer in Bulgaria (Yotov et al., 2013). Severe straining at pre partum due to expulsion of fecal materials, increased intra-abdominal pressure at advanced pregnancy, enlarged rumen making the ligaments fragile around perineum (Ennen et al., 2011), high level of blood estrogen (Miesner and Anderson, 2008), around parturition (Hafez and Hafez, 2000) and deficiency of certain macro or micro minerals may act as potential key factors for vaginal prolapse. Deka et al. (2016) described therapeutic management of pre-partum vaginal prolapse in a jersey crossbred cow. Patra et al., (2014) reported that successful surgical management of cervico-vaginal prolapse can be achieved by retention suture or Buhner’s suture technique. Vaginal prolapse is an emergency condition and it should be treated immediately before there may occur any trauma or laceration to prevent haemorrhage or bacterial infection. Sometimes the prolapse may cause infertility in subsequent pregnancy.
Kuijlaars et al. (2012) said that the disorder as acute one when it sustain for prolong duration and turned into fibrosis resulting impossible in retention to its normal position. The cure of vaginal prolapse depends on early diagnosis, immediate replacement of vagina and assurance of further recurrence (Bhattacharyya et al, 2012). Although there is no published paper in Bangladesh regarding vaginal prolapse. The aim of this report is to present a case of vaginal prolapse in cross breed HF with its diagnosis, treatment and successful management.
History and Clinical Examination
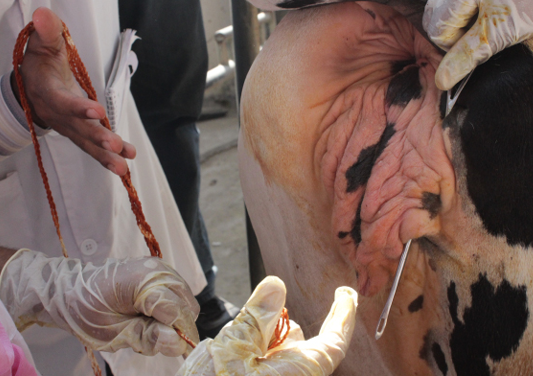
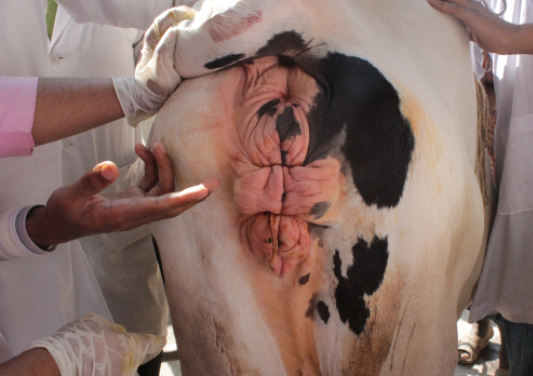
A 6 years old 7 months pregnant cow was admitted to Teaching Veterinary Hospital (TVH) at Chittagong Veterinary and animal Sciences University (CVASU). The cow weighed 300kg. The owner claimed that they have seen a mass coming out through the genital opening 4 days before. They hold the mass by hands and kept it to its normal position. But it recurred. At first we did the general physical examination e.g. temperature, heart rate, pulse rate. Then we examined the hanging mass of cow in standing condition and we confirmed it as vaginal prolapse. The prolapsed mass was swollen and edematous (Figure 1). The cow exhibited signs of restlessness. After that blood sample was collected into two vacutainers with and without anticoagulant for estimation of serum calcium, serum magnesium, serum phosphorus, Hb%, PCV, ESR, etc.
Treatment and Management
At first the perineal region of cow was washed with clean water. Then low epidural anesthesia was done at 1st intercoccygeal space using local anesthetic, 2% lignocaine hydrochloride (5ml) (Figure 2) to prevent straining, easy control of tail and desensitization of pelvic region which facilitate easy manipulation of vagina into its original position. Then prolapsed mass was cleaned with normal saline and mild potassium permanganate solution (Figure 3) to remove dirt. The prolapsed mass was lifted with both hands and replaced to vagina by using thumb fist. Then 2% lidocaine hydrochloride was administered subcutaneously into the lips of vagina where the gerlach needle was punctured (Figure 4). Buhner’s suture (Figure 5) was applied with rope parallel to the vulva apart from vagina keeping one hand space for easy urination. It was suggested to keep the suture for 1 month and then removed it. The owner was told to take proper post-operative care for preventing recurrence. The cow was followed for next 2 months. It recovered (Figure 6) successfully without any complication and delivered a health female calf (Figure 7) normally after completing her gestation period.
As post-operative care we administered DNS 5% (1 litre/i/v/ day) for 5 days, calcium borogluconate [Inj. Cal-D-Mag (Reneta pharmaceuticals) 500ml/day/i/v] for 5 days, Drotaverine hydrochloride [Inj. No-spa 20ml/day/i/m (Ambee Pharmaceuticals)] for 5 days. For topical application at vulvar lips 5% Povidin iodine [Ointment viodin (Square pharmaceuticals) was recommended.

Figure 6: Cow after correction
Discussion
Vaginal prolapse in cow is a hereditary, chronic, recurrent disorder. It is one of the reproductive disorders which cause economic loss to dairy farming by hampering the cows and their milk production. It is mostly occurs in pluriparous animal than heifers (Noakes et al., 2001) during 3rd trimester of pregnancy which is similar to this report. The actual cause of vaginal prolapse is unknown. A combination of increased estrogens levels with decreased progesterone and production of relaxin and specially in the last two weeks of pregnancy (Henricks et al., 2011), may cause relaxation of the pelvic ligaments and surrounding soft tissue structures (Hafez and Hafez, 2000). These changes along with increased intra-abdominal to expel out fecal materials pressure play crucial role in the vaginal prolapse. In this case high intra-abdominal pressure was also recorded which may due to sudden change in feeding habit.

Figure 7: Delivering healthy female calf
Table 1: Hematological and biochemical blood parameters
|
Name of the test |
Result |
Normal Range |
|
S. Calcium |
6 |
8.9-11.7 mg/dl |
|
S. Magnesium |
2.9 |
2.8-3.6 mg/ dl |
|
S. Phosphorous |
3.8 |
4.2-9.1 mg/dl |
|
S.GOT |
168 |
167-513 U/ L |
|
S.GPT |
13.15 |
6-19 U/ L |
|
Hemoglobin |
9 |
8-12 gm% |
|
ESR (Wintrobe tube method) |
2 |
2-5 (mm in 1st hour) |
|
Total count of RBC |
9 |
8-18 million/ cumm |
|
Total count of WBC |
10 |
8-12 thousand/ cumm |
|
PCV |
30 |
50-70% |
|
Lymphocytes |
62 |
50-75% |
|
Neutrophils |
30 |
30-48% |
|
Eosinophils |
8 |
1-8% |
|
Monocytes |
4 |
0-4% |
|
Basophils |
1 |
0-1% |
From biochemical blood analysis it was seen that estimated value of serum calcium and phosphorus was somewhat less than the reference (Table 1) value. This might be a reason of prolapse which supports the finding of Akhtar et al. (2008). We applied Bhuner’s suture technique which found to be most successful technique for preventing further recurrence. There may be happen some unusual sequels e.g. vascular disturbance, trauma, myiasis and fecal contamination of the prolapsed mass. These contaminations may turn into septicemia. However, in this case careful removal of manure and dirt materials and use of mild potassium permanganate solution prevented the uterine or vaginal infection which is also noticed by Prakash et al. (2016). Sometimes in delayed case it is uncorrectable due to excess edema or fibrosis. In this case we faced no difficulty in correction. There happened no recurrence and the cow delivered a healthy female calf.
Vaginal prolapse mainly appear at last trimester of gestation. Diagnosis and treatment of vaginal prolapse is very much important task. Delayed in correction may cause some critical condition such as edema, fibrosis, necrosis, septicemia, myiasis. So the farmers and veterinarian should be careful about early diagnosis, correction and management issues for recovery of the condition which will save the cow from life-threatening condition.
ACKNOWLEDGMENT
The authors were greatly acknowledged to Teaching Veterinary Hospital (TVH), Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh.
CONFLICT OF INTERESTS
No conflict of interest
AUTHORS’ CONTRIBUTION
All authors were contributed equally.
REFERENCES