Research Journal for Veterinary Practitioners
Research Article
A Study on the Prevalence and Etiology of Joint Ill in Calves of Cross-Breed Dairy Cattle in Six Dairy Farms of Bangladesh
Mohammad Shah Jalal1*, Avijit Dutta1, Kazi Muhammad Fakhrul Islam2, Jabin Sultana2, Md. Shahriar Hasan Sohel3, Abdul Ahad1
1Department of Microbiology and Veterinary Public Health; 2Department of Physiology, Biochemistry and Pharmacology; 3Department of Anatomy and Histology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong-4225, Bangladesh.
Abstract | During the study, the proportionate prevalence of joint ill was 2.47% in studied population. The incidence of joint ill was higher in female (57.14%) and calves having 75% Holstein Friesian (HF) blood (71.43%) in compare with male (42.86%) and calves having 62.5% HF blood (28.57%) respectively. During May to July the calves were more (71.43%) prone to joint ill in compare with that of February to April (28.57%). Navel ill was the most important (85.72%) predisposing factor for the joint ill infection. During study, a combined treatment with SP Streptomycin and Penicillin (SP) followed by Kanamycin, Colistin Sulfate, Neomycin and Dexamethasone (KCND) was found significantly (P<0.05) more (75%) efficient than treatment with SP only (0%). The isolated organisms from joint fluid were Escherichia coli and Staphylococcus sp. E. coli isolates were resistant to Ampicillin, Nalidixic acid, Chloramphenicol and Colistin sulphate whereas Staphylococcus sp isolates were resistant to Streptomycin, Nalidixic acid, Chloramphenicol and Colistin Sulfate. Both types of organism were sensitive to Tetracycline and Doxycycline. The mortality of joint ill infected calves was found 57.14%. So, prevention of joint ill through avoiding the predisposing factors and treatment of the affected animals with specific antibiotics after Cultural Sensitivity (CS) test can be recommended.
Keywords | Prevalence, Joint ill, CS-test, Escherichia coli, Staphylococcus sp., Navel ill
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | November 02, 2016; Accepted | October 12, 2016; Published | October 25, 2016
*Correspondence | Mohammad Shah Jalal, Department of Microbiology and Veterinary Public Health, Chittagong Veterinary and Animal Sciences University (CVASU), Khulshi, Chittagong-4225, Bangladesh; Email: shah.jalal.baty@gmail.com
Citation | Jalal MS, Dutta A, Islam KMF, Sultana J, Sohel MSH, Ahad A (2017). A study on the prevalence and etiology of joint ill in calves of cross-breed dairy cattle in six dairy farms of Bangladesh. Res. J. Vet. Pract. 4(4): 66-70.
DOI | http://dx.doi.org/10.14737/journal.rjvp/2016/4.4.66.70
ISSN | 2308-2798
Copyright © 2017 Jalal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In Bangladesh, the total cattle population is about 23.4 million of which 6.14 million is dairy cow (Banglapedia, 2014). The mortality of calves less than one year of age is about 9% (Debnath, 1990) and 20% calf mortality may reduce the net profit of farm up to 40% (Singh et al., 2009). In commercial farming, besides the getting of a calf per dam per year, it is recommended to ensure the survivility of new born animals. But, unfortunately, most of the animals die at young age due to different infectious diseases and surgical disorders like Umbilical hernia, Atresia ani, Navel ill, Gangrenous mastitis, Teat obstruction, Teat crack, Arthritis and Lameness (Hossain et al., 1986; Samad et al., 2002). Prevalence of arthritis in cattle is about 6.1% in Bangladesh (Sarker et al., 2014). Joint ill, also known as arthritis, is one of the significant causes of lameness in different farm animals. It is mostly found in cattle calves, buffalo calves, foals, lambs, kids and piglets etc. (Chakrabarti, 2003). The usual cause arises from infection of the navel, or, less commonly, tailing and castrating wounds, by pathogenic bacteria from a contaminated environment (Angus, 1991). Most commonly joint ill is caused by bacteria, but other microorganisms (virus, fungi and protozoan) are not ruled out with the current standard diagnostic work up (Nuss, 2012). Treatment of joint ill is a challenging fact. For successful treatment of joint ill, early diagnosis is essential. If caught early, antibiotic treatment can be successful (Robson, 2003). When joint ill develops, most of the clinician treats the calf with broad-spectrum antibiotics. The choice of antibiotic will depend on the causative bacteria (Robson, 2003). So, we wanted to know the present status of this economically important disorder in dairy farm of Bangladesh.
Materials and Methods
Preliminary Survey
A preliminary survey was conducted in December 2015 at ten commercial dairy farms from different districts of Bangladesh. The data of last one year were collected using a standard questionnaire. On the basis of survey findings, six farms were selected for a prospective study on calf diseases especially joint ill in calves of crossbred dairy cattle during February to July 2016.
Animal
The total cattle of selected six farms were 800. All the cattle were crossbred dairy cattle. A group of 283 calves having an age of one year or less were considered as study population.
Sample Collection
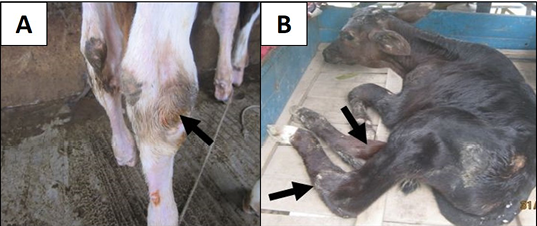
During study period, about 85 calves were diagnosed as cases belonging to different diseases on the basis of clinical signs (Figure 1), physical examination and response to the treatment. Data of 283 calves were collected from different registers and direct survey using a standard questionnaire at weekly interval. Joint fluids from knee joint and/or hock joint of 7 infected calves were collected as sample for bacterial isolation and send to Microbiology laboratory of Chittagong Veterinary and Animal Sciences University (CVASU) and Poultry Research and Training Centre (PRTC), Chittagong, Bangladesh.
Microbiological Analysis
Bacterial isolation was done following a method described by Feng et al. (2011). Peptone broth (Oxoid Ltd., PH: 6.2±0.0), Nutrient broth (Oxoid Ltd., PH: 7.4±0.2), MacConkey agar (Oxoid Ltd., PH: 7.4±0.2) and EMB agar (Merck, PH: 7.1±0.2), Mannitol salt agar (Merck, PH: 7.4±0.2) (Figure 2), Blood agar (Oxoid Ltd., PH: 7.3±0.2) were used for isolation of the organism. The suspected colonies were confirmed on the basis of colonial morphology, Gram’s staining property and biochemical property (Figure 2). After confirmation of isolates as E. coli and Staphylococcus sp., Cultural Sensitivity test of the isolates was determined by using the micro disc diffusion method (Kirby-Bauer method), and the method was used according to guidelines established by Clinical and Laboratory Standards Institute (CLSI, 2015) with minor modification. Muller Hinton agar (Biotec, PH: 7.3±0.1) was used for Cultural sensitivity test. Antibiotics selected for susceptibility testing included a panel of 8 antimicrobial agents (Table 1) of interest to the large animal practitioners to treat joint ill infected calves in studied farms. Measurement of the growth inhibition zone permitted the classification of each isolates as sensitive, intermediate and resistant according to data provided by Oxoid Ltd., Basingstoke, Hampshire, England and CLSI (2015).

Figure 2: A) E. coli colonies with metallic sheen on EMB agar; B) Staphylococcal colonies on Mannitol-salt agar; C) Gram negative E. coli; D) Gram positive Staphylococcus sp.; E) Positive indole test for E. coli; F) Positive Catalase test for Staphylococcus sp.)
Statistical Analysis
All the collected data relating to 283 calves along with data related to joint ill infected calves were checked manually for obvious inconsistencies, recording errors or missing data. Then, data were entered in Microsoft Excel 2007 and descriptive statistics was done by using the STATA-13 software with 95% confidence interval and 5% level of significance. Results were expressed as percentages of different variables.
Table 1: Diameter (zone of inhibition) standards for E. coli and Staphylococcus sp. (CLSI, 2015)
|
Groups of antimicrobial |
Antimicrobial agents |
Disc content |
Zone of inhibition (diameter in mm) |
Manufacturers |
||
|
R |
I |
S* |
||||
|
β –lactams |
Ampicillin (AMP) |
10μg |
≤13 |
14-16 |
≥17 |
Oxoid Ltd. Basingstoke Hampshire England |
|
Aminoglycosides |
Streptomycin (S) |
10μg |
≤11 |
12-14 |
≥15 |
|
|
Neomycin (N) |
30μg |
≤13 |
14-15 |
≥16 |
||
|
Tetracycline |
Tetracycline (TE) |
30μg |
≤14 |
15-18 |
≥19 |
|
|
Doxycycline (DO) |
30μg |
≤10 |
11-13 |
≥14 |
||
|
Quinolones |
Nalidixic acid (NA) |
30μg |
≤13 |
14-18 |
≥19 |
|
|
Chloramphenicol |
Chloramphenicol (C) |
30μg |
≤12 |
13-17 |
≥18 |
|
|
Polypeptide antibiotics |
Colistin sulphate (CT) |
10μg |
≤19 |
20-22 |
≥23 |
|
S*, Sensitive; I, Intermediate sensitive; R, Resistant
Results and Discussion
The prevalence of joint ill in calves of crossbred dairy cattle was about 2.47% during study period of February to July 2016 (Table 2), which is lower than the reported prevalence of Wudu et al. (2008) which was 6% in Ethiopia. It might be due to geographical differences. Joint ill was more prevalent (3.31%) during May to July than that of February to April (0.98%) (Table 2). According to this study, the occurrence of joint ill was higher in female calves (57.14%) than male calves (42.86%) (Table 3), which is supported by the research findings of Goodarzi et al. (2015). The calves having 75% Holstein Friesian (HF) blood were more (71.43%) prone to joint ill in compare with calves having 62.5% Holstein Friesian (HF) blood (28.57%) (Table 3). The mortality of calves infected with joint ill was about 57.14% (Table 3). Mortality was insignificantly (P>0.05) higher in male (66.67%) than in female (50%) (Table 3). Again, mortality due to join ill was more in calves having 75% HF blood (60%) in compare with calves having 62.5% HF blood (50%) (Table 3), but the difference was not statistically significant (P>0.05).
Table 2: Prevalence of different diseases according to different month categories
|
Traits |
No of case N (%) |
Prevalence (%) |
||
|
Feb 16 to Apr 16 |
May 16 to Jul 16 |
Overall |
||
|
URT |
27(31.76) |
10.78 |
8.84 |
9.54 |
|
Pnumonea |
12(14.12) |
5.88 |
3.31 |
4.24 |
|
Diarrhea |
16(18.82) |
8.82 |
3.87 |
5.65 |
|
Dysentery |
6(7.06) |
1.96 |
2.21 |
2.12 |
|
Acidosis |
3(3.53) |
1.96 |
0.55 |
1.06 |
|
Navel ill |
8(9.41) |
2.94 |
2.76 |
2.83 |
|
Joint ill |
7(8.24) |
0.98 |
3.31 |
2.47 |
|
Other |
6(7.06) |
3.92 |
1.10 |
2.12 |
|
Total |
85 |
37.24 |
25.95 |
30.04 |
%, Percentage; URT, Upper respiratory tract infection
Table 3: Overall statistics of joint ill infected calves
|
Traits |
No of case attended |
Fate of The Animals |
Chi-square Value |
P-value |
|
|
Death N (%) |
Recovery N (%) |
||||
|
a) Sex |
|||||
|
Male |
3(42.86) |
2(66.67) |
1(33.33) |
||
|
Female |
4(57.14) |
2(50) |
2(50) |
0.19 |
0.66 |
|
b) Blood percentage |
|||||
|
25%SL x75%HF |
5(71.43) |
3(60) |
2(40) |
||
|
37.5%SL x62.5%HF |
2(28.57) |
1(50) |
1(50) |
0.05 |
0.81 |
|
c) Month categories |
|||||
|
Feb15-Apr15 |
2(28.57) |
1(50) |
1(50) |
||
|
May15-Jul15 |
5(71.43) |
3(60) |
2(40) |
0.05 |
0.81 |
|
d) Treatment |
|||||
|
SP |
3(42.86) |
3(100) |
0(0) |
||
|
SP+KCND |
4(57.14) |
1(25) |
3(75) |
3.93 |
0.04 |
|
e) History of other condition |
|||||
|
Navel ill |
6(85.72) |
N/D |
N/D |
||
|
Amputation of extra leg |
1(14.28) |
N/D |
N/D |
N/D |
N/D |
|
f) Fate |
|||||
|
Recovery |
3(42.86) |
N/D |
N/D |
||
|
Death |
4(57.14) |
N/D |
N/D |
N/D |
N/D |
%, Percentage; SL, Shahiwal; HF, Holstein Friesian; SP, Streptomycin and penicillin; KCND, Kanamycin, Colistin Sulfate, Neomycin and Dexamethasone; N/D, Not Done
About 57.14% joint ill infected calves were subjected to treatment with SP followed by KCND while about 42.86 % were subjected to treatment with SP only. The treatment with SP followed by KCND was more efficient (75%) in compare with treatment with SP only (0%) (Table 3). The difference of fate of animal between two treatment groups was statistically significant (P<0.05). The isolated organisms from the joint fluid sample were E. coli and Staphylococcus sp. The result coincides with the results found in a research conducted by Nuss (2012). E. coli were sensitive to Tetracycline and Doxycycline and fully resistant to Amphicillin, Nalidixic acid, Chloramphenicol and Cholistin Sulfate (Table 4) which support the research findings of Paul et al. (2010). Staphylococcus sp., isolates showed distinctly sensitivity to Tetracycline and Doxycycline, moderately sensitive to Neomycin and resistance to Nalidixic acid, Chloramphenicol, Cholistin sulphate and Streptomycin (Table 4). Recent study findings partially support the previous research findings of Pereira and Siqueira-Jr (1995). The difference might be due to geographical differences or species variation of organism.
Table 4: Results of CS-test for E. coli and Staphylococcus sp. isolates
|
Organism |
Sample No. |
Antimicrobial disc used |
|||||||
|
AMP |
S |
N |
TE |
DO |
NA |
C |
CT |
||
|
JF1 |
R |
I |
I |
S* |
S* |
R |
R |
R |
|
|
E. coli |
JF3 |
R |
I |
I |
S* |
S* |
R |
R |
R |
|
JF6 |
R |
R |
I |
S* |
S* |
R |
R |
R |
|
|
Staphylococcus sp. |
JF2 |
I |
R |
I |
S* |
S* |
R |
R |
R |
|
JF7 |
S* |
R |
I |
S* |
S* |
R |
R |
R |
|
AMP, Ampicillin; S, Streptomycin; N, Neomycin; TE, Tetracycline; DO, Doxycycline; NA, Nalidixic acid; C, Chloramphenicol; CT, Colistin sulphate; S*, Sensitive; I, Intermediate sensitive; R, Resistant
Conclusion
The proportionate prevalence of joint ill has made it as a major animal health issue. As both gram positive and gram negative bacteria contribute to joint ill, and, also, they show resistance to different commonly used antibiotics, so, a complex combination of antibiotic treatment after cultural sensitivity test may be an option of treatment aiming to reduce calf mortality caused by joint ill in dairy farm.
Acknowledgement
Authors would like to express a humble gratitude to all farm owners, field veterinarians and academicians of Chittagong Veterinary and Animal Sciences University, Bangladesh, for their co-operation.
Conflict of Interest
There is no conflict of interest.
Authors’ Contribution
MS Jalal designed the study, carried out lab work and wrote the manuscript. A Dutta analyzed data edited the manuscript. KMF Islam, J Sultana and MSH Sohel collected samples and related data. A Ahad supervised all the activities of this study.
References