Research Journal for Veterinary Practitioners
Case Report
A Clinical Case of Severe Enteric Colibacillosis in a Doe and its Pathological Findings: A Case Report
Faez Firdaus Abdullah Jesse1*, Eric Lim Teik Chung1, Siti Nadhirah Latif1, Yusuf Abba2, Abdulnasir Tijjani1, Muhammad Abubakar Sadiq1, Konto Mohammed1, Lawan Adamu3, Idris Umar Hambali1, Asinamai Athliamai Bitrus2, Annas Salleh2, Mohd Azmi Mohd Lila2, Abdul Wahid Haron1, Abdul Aziz Saharee1
1Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 2Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 3Department of Veterinary Medicine, Faculty of Veterinary Medicine, University of Maiduguri, PMB1069, Borno State, Nigeria.
Abstract | This case reports the clinical management of enteritic collibacillosis in a doe due to E. coli infection. An adult Jamnapari doe weighing 50kg was presented with a primary complaint of inappetance and weakness. The doe was weak, and on sternal recumbency when presented to the University Veterinary Hospital, Universiti Putra Malaysia. Physical examination findings showed pyrexia at 41.0°C, 5% dehydration, capillary refill time of more than 2 seconds, presence of light brown pasty fecal staining around the perineum region and well-formed feces covered with orange mucoid discharge. Bacteriology isolation and identification revealed E. coli from the fecal samples. Therefore, based on the physical examination findings and laboratory result, the case was diagnosed as enteric colibacillosis. The doe was treated promptly and vigorously with fluids, antibiotic, non-steriodal anti-inflammatory drug, and supplement for enteric colibacillosis. However, the doe was euthanized after 4 days of post hospitalization due to poor prognosis. Gross post mortem findings include presence of transparent thinning of the small and large intestinal wall indicative of necrotizing intestinal layer; and petechial haemorrhages in the mucosa of both the small and large intestines. Histopathological findings in the small and large intestine showed infiltrations of inflammatory cells in the submucosa, loss of architecture in the Crypt of Lieberkuhn and necrosis of the Brunner’s’ glands. Both gross and microscopic pathological findings were suggestive of bacterial enteritis. Prompt diagnosis of cases of bacterial enteritis is paramount in order to avoid economic losses associated with the disease.
Keywords | Enteric colibacillosis, doe, clinical management, gross pathology, histopathology
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | May 16, 2016; Accepted | May 24, 2016; Published | June 08, 2016
*Correspondence | Faez Firdaus Abdullah Jesse, Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; Email: jesseariasamy@gmail.com
Citation | Jesse FFA, Chung ELT, Latif SN, Abba Y, Tijjani A, Sadiq MA, Mohammed K, Adamu L, Hambali IU, Bitrus AA, Salleh A, Lila MAM, Haron AW, Saharee AA (2016). A clinical case of severe enteric Colibacillosis in a doe and its pathological findings: A case report. Res. J. Vet. Pract. 4(2): 34-38.
DOI | http://dx.doi.org/10.14737/journal.rjvp/2016/4.2.34.38
ISSN | 2308-2798
Copyright © 2016 Jesse et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Escherichia coli is a gram negative, rod, facultative anaerobic bacteria that is commonly found in the intestine of humans and animals (Wells et al., 1991; Besser et al., 1997; Fairbrother and Nadeau, 2006). Most E. coli are harmless and are usually normal flora in the intestine. However, some E. coli strains such as shiga toxin-producing E. coli, enterotoxigenic E. coli, enteropathogenic E. coli, enteroaggregative E. coli, enteroinvasive E. coli and diffusely adherents E. coli are pathogenic and cause diarrhea in the host (Wani et al., 2013). These pathogenic strains can be transmitted through contaminated water, food, direct or indirect contact with infected animals or a person (Kahn and Line, 2005; Fairbrother and Nadeau, 2006). Enteric colibacillosis is a common disease in young animals caused by colonization of enterotoxigenic strain of E. coli in the small intestine (Kahn and Line, 2005). Proper hygiene with good husbandry practice in the farm is the key to control and prevent E. coli disease.
Enteritis is an inflammation of the intestinal mucosa resulting in clinical signs of diarrhea, dysentery, abdominal pain, dehydration as well as electrolyte loss and imbalance. In severe case, mucus might be present in the feces while in worst case there might be shreds or sheets of exfoliated mucosa (Blood et al., 2011). The condition depends on the cause, severity and location. According to Ghanem et al. (2012), there are a few predisposing factors causing enteritis. Firstly, newly born kids with deficiency of immunoglobulins are more susceptible than the adults. Second, multiple stresses such as transportation, deprivation of food and water or weaning may cause immunosuppression to the animal thus leading to enteritis. Next, prolonged use of antibacterial agents through oral route may alter the intestinal microflora and permit the development of resistance by the organisms. In brief, all factors causing enteritis lower the immunity of affected animals causing an increase in the pathogenicity of the causative agent (Ghanem et al., 2012). This case report describes the clinical management of severe clinical enteritis in a doe and its pathological findings.

Figure 1: Doe with head tilted to the left flank
Case Report
History
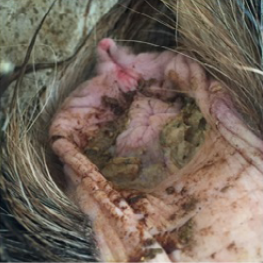
An adult Jamnapari doe weighing 50kg with body condition score of 2 out of 5 was presented to the University Veterinary Hospital, Universiti Putra Malaysia with a primary complaint of inappetence and weakness. During presentation, the doe was weak and in sternal recumbency with the head tilted towards the left flank (Figure 1). The doe had a history of kidding two days prior to presentation. Vaccination and deworming were not practiced in the semi-intensive farm.
Physical Examination
Physical examination findings were pyrexia (41.0°C), 5% dehydration, capillary refill time of more than 2 seconds, presence of light brown pasty fecal staining around the perineum region and well-formed feces covered with orange mucoid discharge (Figure 2 and 3).
The differential diagnoses were coccidiosis, parasitic gastro-enteritis (PGE) and bacterial enteritis.
Laboratory Investigation and Results
Haematology findings revealed a normochromic normocytic anaemia with packed cell volume of 0.20L/L, which may be due to the previous parturition associated haemorrhage; and leukopaenia (1.95x109/L), which was suggestive of immunosuppression. The serum biochemistry showed hypoalbuminaemia (20.1g/L), which may be due to malnutrition and protein losing enteropathies. Besides that, fecal sample was collected for parasitology and bacteriology identification. For parasitology identification, Modified McMaster Fecal Egg Count Technique was done and the result was negative for strongyle and coccidian ova. However, for bacteriology identification and isolation, the fecal sample was positive for E. coli. Thus, the final diagnosis for this case was enteric colibacillosis.
Treatment
The doe was treated promptly and vigorously for enteric colibacillosis. The dehydration was corrected by intravenous infusion of Lactated Ringer’s solution administered for three days. Oxytetracycline 20mg/kg was injected intramuscularly once as broad-spectrum antibiotic to treat E. coli infection. Flunixin meglumine 2.2mg/kg was administered intravenously TID for three days as analgesic, anti-inflammatory and anti-pyrexia. 10mL of Kaolin-Pectin was given orally BID for two days to solidify the loose feces. 5mL of Biodyl was also administered intramuscularly once as vitamin supplement. Furthermore, physiotherapy was also performed twice daily to improve blood circulation and muscle functions.
Progression
The condition of the doe was not improving with persistent head tilt and frequent vocalization due to pain. Hence, the doe was euthanized using Xylazine 2% and Pentobarbital 200mg/kg, intravenously on Day 4 of hospitalization. The carcass was then sent for necropsy examination.
Pathology Findings
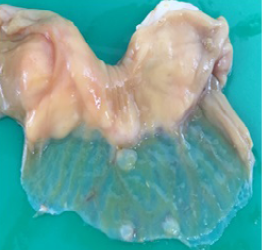
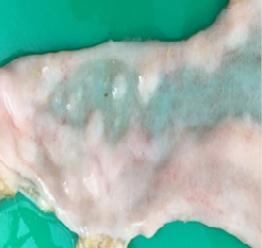
Gross pathology findings showed presence of transparent thinning of the small and large intestinal wall indicative of necrotizing enteritis (Figure 4), and petechial haemorrhages in the mucosa of both small and large intestines (Figure 5). Histopathological examination of the small and large intestines showed infiltration of inflammatory cells in the submucosal layer, loss of architecture in Crypt of Lieberkuhn and necrosis of the Brunner’s gland (Figure 6).
Final Diagnosis
Based on the physical examination, diagnostic work-ups, post mortem and histopathological findings, the final diagnosis made was E. coli associated enteritis.
Discussion
Bacteria, viral, protozoa and fungal are few virulent agents that may cause enteritis in small ruminants such as sheep and goat (Kahn and Line, 2005). In this case, the isolated bacteria were E. coli which caused enteric colibacillosis in an adult doe. However, colibacillosis is a major cause of diarrhea in kids. There are four main type of E. coli that can potentially be associated with diarrhea in goat kids; these are enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC) and necrotoxigenic E. coli (NTEC). Nonetheless, enteropathogenic E. coli (EPEC), which also known as adhering and effacing E. coli (AEEC) is usually the causative agent for severe diarrhea in both kids and adult sheep and goat (Wani et al., 2013).
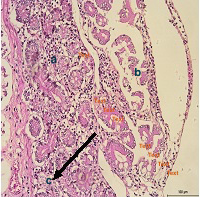
Figure 6: Infiltrations of inflammatory cells in the submucosa layer; b) loss of architecture in Crypt of Lieberkuhn; c) necrosis of the Brunners' gland in the small and large intestine
There are several common clinical signs that can be seen in the animal having enteritis; these include diarrhea, dehydration, weight loss, systemic reaction, acid-base and electrolyte imbalance as well as abdominal pain which were observed in this case (Blood et al., 2011). In the present case, if euthanasia was not done, the cause of death in the doe may be due to circulatory failure because of the severe dehydration. Inflammation and necrosis of the intestinal wall, efficiency of fluid absorption and excretion in the intestine will be reduced significantly. E. coli produces enterotoxins which are virulent factor that adhere to the enterocytes through the use of frimbae and cause severe infection and inflammation in the submucosal layer of the intestine (Kaper et al., 2004). As observed in this case, severely dehydrated animals will have electrolyte imbalances and metabolic acidosis causing hyperventilation and muscle weakness due to inadequate bicarbonate and sodium concentrations in the blood circulation (Ghanem et al., 2012).
The primary therapeutic plan for animals with enteric collibacillosis is usually vigorous antimicrobial therapy to reduce morbidity and mortality (Wani et al., 2013). In the present case, Oxytetracycline 20mg/kg was used as the antibiotic of choice, but the doe did not response well to the treatment. According to Bogaard et al. (2000) and Wani et al. (2013), the wide usage of antibiotics had caused the development of antimicrobial resistance in many types of pathogenic E. coli and this may be the reason for poor response in the doe. Abu Daud et al. (2014) had stated that, polymerase chain reaction can be used in detection of antibiotic resistance in some E. coli strains, but this was not performed in this case.
Conclusion
Enteric collibacillosis may occur in animals of any age due to E. coli infection where common clinical signs such as diarrhea, dehydration, weight loss, and abdominal pain will be observed. The disease can be prevented with proper farm hygiene and can be control by early diagnosis and prompt treatment to prevent great economic losses in the ruminant industry.
Acknowledgement
The authors wish to acknowledge the supporting staff of University Veterinary Hospital, University Putra Malaysia in particular Mr Mohd Jefri Norsidin and Mr Nazim Razali Kanani for their technical help and support.
conflict of interests
There exists no conflict of interest.
Authors’ contribution
All authors contributed equally to the work and are responsible for the data presented herein.
References