Research Journal for Veterinary Practitioners
Research Article
Haematological Studies on Stunting Syndrome in Broilers
Muhammad Fiaz Qamar, Hina Aslam, Ayesha Liaqat
Department of Zoology, Government College University Lahore, Pakistan.
Abstract | Runting Stunting Syndrome (RSS) is a multifactorial disease with many names and faces that had caused considerable economic losses to poultry through reduced uniformity, reduced livability, decreased body weights, elevated feed conversions and many secondary diseases. The aim of current study was to evaluate the effect of stunting syndrome on haematology in chicks (n = 120) of different ages collected from nine (9) different farms. Grouping was done on the basis of age (G1 = 1–10 days, G2 = 11–20 days, G3 = 21–30 days, G4 = 31–40 days) including both stunted and normal chicks. Haematological results exhibited lower levels of haemoglobin (Hb), Total Erythrocyte Count (TEC) and Total Leucocyte Count (TLC) as compared to normal. Absolute Lymphocyte Count (ALC), absolute monocyte count (AMC) and Absolute Eosinophil Count was also found lower than normal. All these changes may interfere with normal digestive processes and normal body functioning resulting in poor weight gain and retarded growth or stunting of chicks.
Keywords | Runting stunting syndrome, Haematological studies
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | July 12, 2014; Revised | August 15, 2014; Accepted | August 18, 2014; Published | November 13, 2014
*Correspondence | Muhammad Fiaz Qamar, Government College University Lahore, Pakistan; Email: dr.fiazqamar@gcu.edu.pk
Citation | Qamar MF, Aslam H, Liaqat A (2015). Haematological studies on stunting syndrome in broilers. Res. J. Vet. Pract. 3 (1): 19-24.
DOI | http://dx.doi.org/10.14737/journal.rjvp/2015/3.1.19.24
ISSN | 2308-2798
Copyright © 2015 Qamar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Runting Stunting Syndrome (RSS) is a condition in which members of a flock appear with relatively smaller bodies due to retarded growth (Zavala and Sellers, 2005). Smaller than normal feathers may appear on affected chicks that are usually curled at wing tips; therefore, the disease is referred as Helicopter disease. The beaks and legs of effected birds may sometimes appear pale in colour and we name it pale bird syndrome. Not all but some of the affected birds may have rickets, weak or broken legs and the syndrome is called Brittle bone disease (Clarck and Jones, 2008). The syndrome is sometimes characterized by the more chances of occurrence (2–15%) in birds of two weeks of age with reduced body weight and poor feathering (Smart et al., 1988).
There are large number of possible reasons for RSS including some management issues, environmental challenges, infectious diseases, faulty poultry genetics, nutrition and feeding, and perhaps some other reasons and/or any possible combinations of all these (Zavala and Sellers, 2005; Qamar et al., 2013). Some particular chicks were observed more susceptible than others and the males were found affected much severely than females (Rebel et al., 2006).
Although the disease may be infectious in nature yet some non–infectious agents cannot be ignored. Infectious agents mostly viruses have been isolated as etiological agents, specifically rotaviruses, reoviruses, astroviruses and some other small rounded viruses present in the intestines and intestinal contents of infected chicks by using some isolation techniques or direct electron microscopy, (Zavala and Barbosa, 2006). Bacteria often isolated from the intestines of RSS affected birds often cause secondary infections (Clarck and Jones, 2008).
Appearance and disappearance of RSS is equally sudden making difficult to decide about the effective control measures. Although there is no satisfactory treatment or vaccination for this disease yet but proper poultry house management, strict hygienic and sanitary measures, biosecurity procedures, good parent nutrition and egg selection reduce the possibility of exposure and incidence of disease.
The haematology of stunted chicks is also affected severely by RSS. Different blood parameters may increase or decrease significantly due to retarded or stunted growth. The overall mean values of mean packed cell volume (PCV), total erythrocyte count (TEC) and haemoglobin (Hb) were reduced significantly in stunting birds, suggesting some anemic changes caused by malabsorption. In contrary no changes were observed in PCV value in birds affected with Malabsorption Syndrome (MAS) as stated by Rani et al. (2011). The TLC value did not differ significantly, however increased values of AMC, ABC and AHC with decreased levels of ALC were indicators of the infectious nature of the syndrome (Turgeon, 1999); therefore paleness of legs and beaks of affected chicks may be due to decreased Haemoglobin.
The aim of current research was to differentiate between the haematological parameters of affected and healthy broilers by Runting Stunting Syndrome, to study the epidemiological factors contributing to the occurrence/causation of syndrome and to develop a strategy for the effective control of RSS.
MATERIALS AND METHODS
Sampling
The study was conducted during the period of January 2012 to July 2012 on the stunted chicks of one to forty days of age. Blood Samples were taken from 120 chicks of 4-40 days of age.
Poultry Rearing
Collected chicks were divided into four groups. Each group contained 30 chicks. Grouping was done on the basis of age. Each group was divided into 3 sub-groups. Six stunted and four normal chicks of same age were kept in each sub group. So, 18 stunted and 12 normal chicks were present in each group. 4, 7 and 10 days old chicks were kept in 1st group (G1: 1–10 days of age). Second group (G2: 11–20 days of age) contained 11, 14 and 19 day old chicks. Third group (G3: 21–30 days of age) contained 22, 26 and 28 day old chicks. 32, 35 and 40 day old chicks were kept in 4th group (G4: 31–40 days of age). Total number of groups, age of chicks and total number of chicks in each age group are given in table 1.
Blood Sampling
Blood sampling of stunted chicks was done on alternate days from alternate groups. These groups included both stunted and normal chicks. Blood volume is 10% of the body weight and amount of blood that can be safely collected is 10% of that blood. Blood samples were collected from blood vessels. The right jugular vein is easily assessable. Other sites of vein puncturing are wing vein (brachial, basilica or cutaneous ulnar). Blood samples were also sometimes obtained by slaughtering birds; the blood was collected by heart puncture (Guyton, 1999; Ganog, 1991). Chicks were slaughtered and blood was collected by 3cc syringe and was stored in EDTA coated blood sampling tubes.
Haematological Studies
By using an automated haematological counter SYSMAX E–5000 fresh blood was tested for haematological studies. Following parameters were studied: Haemoglobin (Hb), Total erythrocyte count (TEC), Total leucocytes count (TLC), Absolute lymphocyte count (ALC), Absolute monocyte count (AMC) and Absolute eosinophil count (AEC) (Sellers et al., 2010).
Statistical Analysis
Mean weights with standard errors were calculated for different groups of RSS affected chicks and normal chicks. Mean of body weights of stunted and normal chicks were compared by using T–test. Percent reduced body weights were also found for stunted chicks. Mean values with standard errors were calculated for each parameter of haematology and their means were compared by T–test. All the data collected was analysed statistically by using software, SPSS 18.0 ver. to calculate using the appropriate statistical techniques.
Table 1: Age–wise grouping of chicks
|
Sr. No |
No. of groups |
Age of chicks (days) |
No. of stunted samples in each group n = 18* |
No. of normal samples in each group n = 12* |
Total number of chicks n = 30 |
Total no. of chicks in each group |
|
1 |
G1 |
4 7 10 |
6 6 6 |
4 4 4 |
10 10 10 |
30 |
|
2 |
G2 |
11 14 19 |
6 6 6 |
4 4 4 |
10 10 10 |
30 |
|
3 |
G3 |
22 26 28 |
6 6 6 |
4 4 4 |
10 10 10 |
30 |
|
4 |
G4 |
32 35 40 |
6 6 6 |
4 4 4 |
10 10 10 |
30 |
|
5 |
G |
1–40 |
72 |
48 |
120 |
120 |
*18 stunted and 12 normal chicks were taken in each group
RESULTS
Chicks that were 1-40 day old were divided into 4 groups. Grouping was done on the basis of age (Table 1). The clinical signs and symptoms stunted syndrome has been shown in figure 2, figure 3, and figure 4.
Haematological Findings
Values for different blood parameters were noted by using a haematological analyzer (sysmax E 5000). RBCs were corrected for NRBCs. Haematological findings were given in table 1 and figure 1.
Haemoglobin (Hb)
Haemoglobin value as noted by the analyser for the normal chicks was 11.0±0.21 (g/dl), while for the stunted chicks this value was found lower 6.93±0.17g (g/dl). The difference in Hb value was found significantly lower for stunted chicks. It may be suggestive of anaemic changes or pale bodies of stunted chicks.
Total Erythrocyte Count (TEC)
Values provided by the analyser for total erythrocyte count for normal chicks was 3.19±0.11 (109)/l while for stunted chicks it was 2.5+11 (109)/l. RBCs in stunted chicks were also found lower than normal.
Total Leucocytes Count (TLC)
Analyser had provided the leukocyte count for normal chicks 28.56±1.8 (1012) while for stunted chicks the leucocytes count was 45.3±1.3 (1012)/l. Stunted chicks were exhibited to have higher values for TLC as compared to normal.
Absolute Lymphocyte Count (ALC)
As noted by the analyser the lymphocytes were observed 35% for stunted chicks. Absolute lymphocyte count for stunted chicks was 16.5±2.03 (1012)/l while for normal chicks ALC was 17.49±0.89 (1012)/l. ALC for stunted chicks was found lower than normal.
Absolute Monocyte Count (AMC)
By using analyser monocytes were estimated 3.97% for stunted absolute monocyte count for stunted chicks was 1.8±0.09 (1012)/l while for normal chicks estimated AMC was 1.49±0.03 (1012)/l. Monocyte count was found higher than normal.
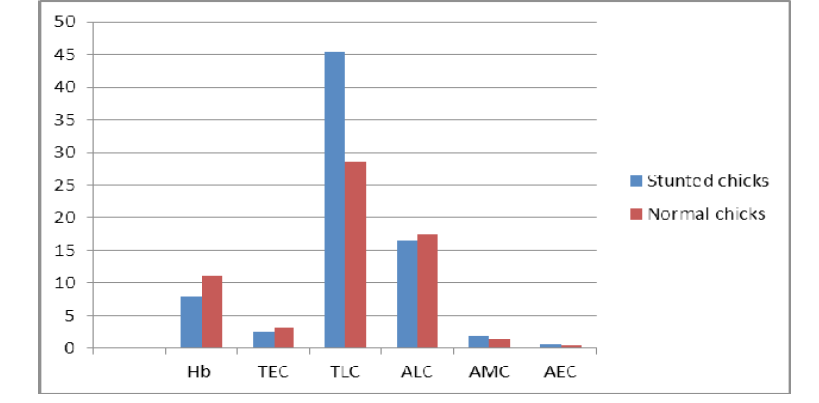
Figure 1: Showing graphical representations of the haematological parameters of Normal and Stunted Chicks

Figure 2: Showing a stunted helicopter chicken
Absolute Eosinophil Count (AEC)
Eosinophils for stunted chicks were estimated (1.5%) with the help of analyzer. Absolute count for eosinophils was observed 0.7±11 (1012)/l for stunted chicks while for normal chicks estimated value AEC was 1.29±011 (1012)/l. AEC was also found lower in stunted chicks than normal.

Figure 5: Showing stunted chick
DISCUSSION
Runting stunting syndrome (RSS) is a complex syndrome of young broiler chickens. It is known by various names as Mal Absorption Syndrome (MAS), Brittle bone disease, infectious proventriculitis and helicopter disease very often characterized in 2–15% birds with lower body weights and poor feathering at about 2 weeks of age (Rani et al., 2011; Jindal et al., 2009).
Haematology or blood study is an essential medical science applied to the diagnosis and treatments of various disorders related to blood. Basic procedures such as complete blood count (CBC) including the measurement of various parameters as platelets, Red blood cells RBCs, (erythrocytes), white blood cells (Leukocytes) and absolute count of eosinophils, basophils, neutrophils, monocytes and lymphocytes. Haemoglobin level, total erythrocyte count, absolute leucocyte count, and absolute eosinophil count were found decreased while total leucocyte count and absolute monocyte count was found higher (Nilli et al., 2007).
Erythrocytes also called red blood cells are highly specialized for their principal function of transport of oxygen and carbon dioxide. RBCs are enucleated biconcave discs elliptical cells with pink cytoplasm and basophilic nuclei.
The fully differentiated erythrocyte consists of an outer plasma membrane enclosing haemoglobin and limited number of enzymes necessary for membrane integrity and gas transport. The binding, transport and delivery of oxygen by haemoglobin are not dependent on erythrocyte metabolism. However erythrocytes utilize energy in maintenance of electrolyte gradients across plasma membrane and Hb in reduced active from. After haemolysis of RBCs, Hb is removed and broken down into haeme and globin. The oven all mean haemoglobin and total erythrocyte count (TEC) were found significantly recued in the blood of stunted chicks. If haemoglobin concentration of red blood cells is below the standard limit then anaemia is considered to be present (Turgeon, 1999). These results were found similar to the finding of Rani et al. (2011) who found the decreased values of Hb and TEC and relate them with anaemic changes caused by malabsorption syndrome.
All living bodies have specialized system for combating toxic and infections agents. Leucocytes (WBCs) comprise the mobile units of body’s protective system (Sellers et al., 2010). They are almost round in shape and are of 5 different types that are traditionally divided into gnanulocytes (Neutrophils, Basophils and Eosinophils) and agranulocytes also called mononuclear leucocytes (Lymphocytes and monocytes) (Riddle and Derow (1985). Leucocytes constitute an important part of body defences against foreign invaders. Generally leucocytes perform their appropriate functions in specific tissues and use the blood just as a vehicle for transit between site of formation, activity and storage. The values for total leucocyte count as obtained in present study were significantly higher than normal are in agreement with the studies of Rani et al. (2011). The increase in WBCs could be due to many reasons including stress and infection (Otto et al., 2006). In present study the infected organisms were found with damaged tissues and release of increased number of leukocyte might be a response to infection or injury.
Basophils are the least common type that contains histamine and heparin in their granules while neutrophils are the most common type of leucocytes in blood. In circulation, the neutrophils are attracted by bacterial infections. Reflecting their increased number in circulation eosinophils are considered haemeostatic regulators of inflammation (Reece, 2011). Eosinophils number was found higher in many types of parasitic disease and defence against these parasites, in present study the eosinophil count was found less than normal as studied by Rani et al. (2011). The reason may be that the mechanism of tissue damaging as determined histopathological was higher as compared to the defences against parasites causing damages.
Monocytes are the largest of white cells. They respond to the presence of invading microorganisms, inflammation by migration into the tissues and necrotic materials (Sellers et al., 2010) lymphocytes are the smallest cells in the white cell series. Their increased number was seen during viral infections also playing part in immunological defence mechanism (Young and Heath, 2000).
The lymphocyte count was found lower indicating that chicks may have a lower immunological defence mechanism but this report could not provide any evidence for infectious agents. The values for AMC were also found higher. The results for ALC were also found similar to the findings of Rani et al. (2011) but the results for AMC were different. The reason may be the variations in environmental or management factors or the infection of chicks due to involvement of different pathogens. Severity of disease and immunity in chicks also varies causing different results.
CONCLUSION
Haematological changes may affect the immunity of RSS affected chicks resulting in poor weight gain and delayed or retarded growth. High cull rates, greater than expected variations in weights, reduced weight for age and sale of small chicks lead to considerable economic losses. Proper vaccination or treatment of affected organs may help reduce the chances of RSS.
REFERENCES






