Journal of Animal Health and Production
Short Communication
Journal of Animal Health and Production 1 (1): 6–9Sero–epidemiological Pattern of Salmonellosis in Animals of Rohilkhand Region, Uttar Pradesh, India
Neelam Sachan1*, Binita Nautiyal1, Rajesh Kumar Agarwal2, Veer Pal Singh3, Amit Kumar Verma4
- Department of Animal Science, Faculty of Applied Sciences, M. J. P. Rohilkhand University, Bareilly, U.P.–243122;
- Indian Veterinary Research Institute, Izatnagar, Bareilly, U.P. 243122;
- Department of Livestock Product Technology, 2Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu ChikitsaVigyan Vishwavidyalaya Ewam Go–Anusandhan Sansthan, Mathura (U.P.) – 281001, INDIA.
- Department of Veterinary Epidemiology and Preventive Medicine, 2Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu ChikitsaVigyan Vishwavidyalaya Ewam Go–Anusandhan Sansthan, Mathura (U.P.) – 281001, INDIA.
*Corresponding author: neelamvet@rediffmail.com
ARTICLE CITATION:
Sachan N, Nautiyal B, Agarwal RK, Singh VP, Verma AK (2013). Sero–epidemiological Pattern of Salmonellosis in Animals of Rohilkhand Region, Uttar Pradesh, India. J Anim. Health Prod. 1(1): 6–9
Received: 2013–04–03, Revised: 2013–05–04, Accepted: 2013–05–07
The electronic version of this article is the complete one and can be found online at
(
http://nexusacademicpublishers.com/table_contents_detail/11/38
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
During the period understudy (2008–10), a total of 1500 serum samples of cattle, buffalo, goats and dogs from five districts of Rohilkhand region, Uttar Pradesh, India were screened for presence of Salmonella agglutinins employing slide micro–agglutination test using two coloured antigens of Salmonella Typhimurium (O–4,5,12) and S. Enteritidis (O–9,12). An overall sero–prevalence of Salmonella agglutinin against O–4,5,12 and O–9,12 antigens in animals was 15.53% and 13.6%, respectively, while overall sero–prevalence of Salmonella agglutinins against both the antigen was 11.4%. Species wise distribution of positive agglutinins against both antigens was highest in dogs (25.2%), followed by cattle (11%), buffalo (9.2%) and goats (2.8%). The prevalence of agglutinins in sera of animals was highest in animals of district Bareilly (13%). In all the animal species, seropositivity was highest during winter season. Sex of animal had no significant effect on sero–prevalence of agglutinins against both Salmonella antigens. Age wise prevalence of positive agglutinins was found to decrease with age in buffalo and goats, while it showed an increasing trend in dogs.
More than 60% diseases of human beings are zoonotic in nature and animals are important source of these diseases including campylobacteriosis, salmonellosis, colibacillosis etc (Verma et al., 2007; Kumar et al., 2012a and 2012b; Dhama et al., 2013). Among these, salmonellosis is one of the most common zoonotic diseases affecting man, animals (Verma et al., 2011a and 2011b) and wide variety of other hosts including reptiles, insects, birds etc. Salmonella may cause a wide spectrum of disease occurrence ranging from sub–clinical, impaired productivity, clinical manifestations that may become often fatal. The infection spreads through contaminated feed and water (Verma et al., 2007) and infected animals tend to shed the organisms in their faeces and secretions (Verma et al., 2008). The isolation of Salmonella from the faecal sample is quite difficult and takes approximately 4–7 days (Verma et al., 2011b). Animal owners, handlers, veterinary health professionals, abattoir workers etc may have chances to get the infection. Salmonellosis, is endemic in India affecting man and animals alike (Raichowdhuri, et al., 1983) but the actual magnitude of disease is not well documented (Niyogi et al., 1999). Therefore, it is necessary to keep watch on prevalence of salmonellosis in animals. As the isolation of Salmonella is tedious and time consuming, use of simple, rapid serological tests like slide micro–agglutination test is convenient method for detecting the prevalence of Salmonella infection in animals
During the period of 2008–10, a total of 1500 blood samples of cattle (725), buffalo (393), goats (180) and dogs (202) attending veterinary hospitals in different districts of Rohilkhand region viz., Moradabad, Rampur, Bareilly, Shahjahanpur and Pillibhit were collected with the help of field veterinarians and their supporting staff. About 5.0ml of blood was collected aseptically using hypodermic syringe and allowed to clot by keeping at room temperature for 3 hours. Serum was separated and kept at –20ºC till use. During sampling, epidemiological data like age, sex of animal and season were also collected.
Slide micro agglutination test was employed to assess the sero–prevalence of Salmonella agglutinins in different animals. For this purpose, two coloured Salmonella antigens O–4,5,12 and O–9,12 were used. Antigen O–4,5,12 belonged to S. Typhimurium group O:4(B) whereas antigen O–9,12 belonged to S. Enteritidis group O: 9(D1). The test was performed as per the method described by Muktaruzzaman et al. (2010). For performance of the test, about 2–3 drops of coloured antigen were placed on a clean grease free glass slide. Then 2–3 drops of test serum were placed adjacent to the antigen. By using a match stick or toothpick, antigens and sera were mixed and observed for 2–3minutes. Appearance of thick clump (agglutination) was taken as a positive result whereas absence of clump formation was indicative of negative test.
The epidemiological data obtained from the study were statistically analyzed by the methods described by Snedecor and Cochran, 1980.
total of 1500 serum samples from different species of animals were screened for presence of Salmonella agglutinin against Salmonella antigen O–4,5,12 and O–9,12. Species wise sero–prevalence of Salmonella agglutinin against Salmonella antigens (O–4,5,12 and O–4,12) were shown in table 1. From the table 1, it can be seen that the overall sero–prevalence of Salmonella agglutinin against O–4,5,12 and O–9,12 antigens in animals were 15.53% and 13.6%, respectively, while an overall sero–prevalence of Salmonella agglutinins against both the antigens was 11.4%. The higher sero–prevalence of Salmonella agglutinin against Salmonella antigen O–4,5,12, which belongs to Salmonella Typhimurium group showed the higher circulation of this serovar in Rohilkhand region, Uttar Pradesh, India. In a sixteen year study, Basu et al. (1975) also reported that S. Typhimurium was the most common serotype in animals in India. Verma et al., 2007 also reported the isolation of Salmonella Typhimurium from apparently healthy dogs of district Bareilly, which is under Rohilkhand region. Since S. Typhimurium can infect man and animals equally, interspecies sharing of this serovar have also been observed (Singh et al., 2010).
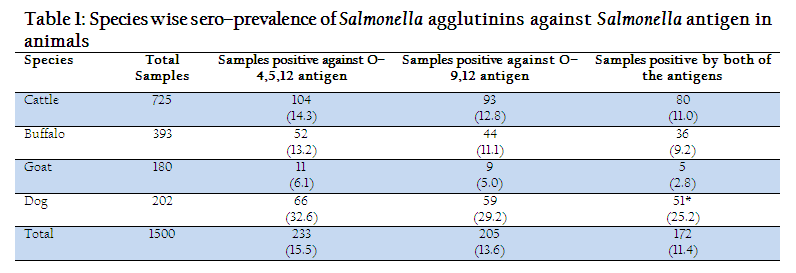
Table 1: Species wise sero–prevalence of Salmonella agglutinins against Salmonella antigen in animals
Species wise distribution of positive agglutinin against both the antigen were significantly higher in dogs (25.2%) in comparison to that of other species viz., cattle (11%), buffalo (9.2%) and goats (2.8%). The possible reasons for higher seroprevalence of Salmonella agglutinins in dogs was there habit of roaming here and there and preferably outside; close association with human beings; and thus increased chances of interspecies transmission (Verma et al., 2008). The effect of species on Salmonella infection has also been reported earlier in various studies. Singh et al. (2010) have also reported higher seroprevalence of salmonellosis in pet dogs than other animals. Contrary to our findings, Chandra et al. (2007) reported 40% seroprevalence of Salmonella antibodies among goats in Bareilly district using MAT ‘O’ test. But the reason for higher seroprevalence could be attributed to higher sensitivity of MAT.
Place wise distribution of Salmonella agglutinins against Salmonella antigen in animals is given in figure 1. The overall sero–prevalence of agglutinins in animal sera were highest in district Bareilly (13.0%), followed by Rampur (11.4%), Shahjahanpur (11%), Pilibhit and Budaun (10% each), and Moradabad (8.6%). From the figure 1, it can also be seen that percentage positive in different animals varied with districts. The possible reason for this variation in different animals might be due to difference in the number of samples in different areas and prevalence of different serotypes in different areas. Verma et al. (2008) have also reported that place wise difference was very much expected as different serovars are prevalent in different areas.
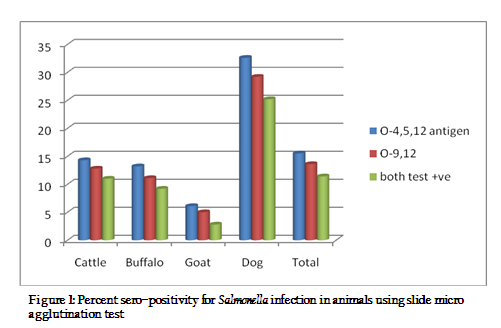
Figure 1: Percent sero–positivity for Salmonella infection in animals using slide micro agglutination test
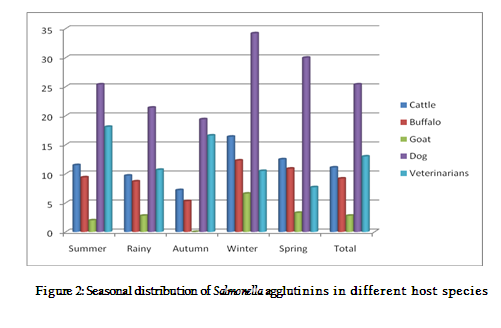
Seasonal distribution of Salmonella agglutinins in different animal species was compared as depicted in figure 2. Results showed that seroprevalence of salmonellosis were significantly higher during winter season in all the animal species in comparison to that of other seasons. Similar pattern was seen in all the species of animals but highest seroprevalence was recorded in dogs (34.2%) during winter season. During winters, animals have low immunity and overcrowding of the animals facilitates the spread of infection.
Sex wise sero–prevalence of Salmonella agglutinins was almost similar in both the sexes of all animal species with minor differences. In agreement to our findings Verma et al. (2008) also reported non–significant difference in sero–prevalence of Salmonella agglutinins between male and females dogs.
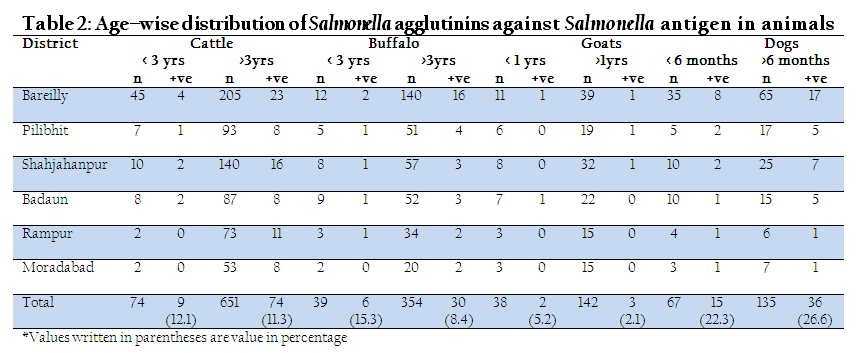
Age wise distribution of seroprevalence of Salmonella agglutinins is shown in table 2. Data obtained in the study showed that in cattle, young (below 3 yrs) as well as adult cattle (>3 yrs) had almost similar sero–prevalence. Young buffaloes had higher sero–prevalence (15.3%) in comparison to that of adult buffaloes (8.4%). Similarly, young goats also had higher seroprevalence (5.2%) in comparison to that of adult (>1 yrs) goats (2.1%).
Species wise distribution of positive agglutinin against both the antigen were significantly higher in dogs (25.2%) in comparison to that of other species viz., cattle (11%), buffalo (9.2%) and goats (2.8%). The possible reasons for higher seroprevalence of Salmonella agglutinins in dogs was there habit of roaming here and there and preferably outside; close association with human beings; and thus increased chances of interspecies transmission (Verma et al., 2008). The effect of species on Salmonella infection has also been reported earlier in various studies. Singh et al. (2010) have also reported higher seroprevalence of salmonellosis in pet dogs than other animals. Contrary to our findings, Chandra et al. (2007) reported 40% seroprevalence of Salmonella antibodies among goats in Bareilly district using MAT ‘O’ test. But the reason for higher seroprevalence could be attributed to higher sensitivity of MAT.
Place wise distribution of Salmonella agglutinins against Salmonella antigen in animals is given in figure 1. The overall sero–prevalence of agglutinins in animal sera were highest in district Bareilly (13.0%), followed by Rampur (11.4%), Shahjahanpur (11%), Pilibhit and Budaun (10% each), and Moradabad (8.6%). From the figure 1, it can also be seen that percentage positive in different animals varied with districts. The possible reason for this variation in different animals might be due to difference in the number of samples in different areas and prevalence of different serotypes in different areas. Verma et al. (2008) have also reported that place wise difference was very much expected as different serovars are prevalent in different areas.
Seasonal distribution of Salmonella agglutinins in different animal species was compared as depicted figure 2. Results showed that seroprevalence of salmonellosis were significantly higher during winter season in all the animal species in comparison to that of other seasons. Similar pattern was seen in all the species of animals but highest seroprevalence was recorded in dogs (34.2%) during winter season. During winters, animals have low immunity and overcrowding of the animals facilitates the spread of infection.
Sex wise sero–prevalence of Salmonella agglutinins was almost similar in both the sexes of all animal species with minor differences. In agreement to our findings Verma et al. (2008) also reported non–significant difference in sero–prevalence of Salmonella agglutinins between male and females dogs.
Age wise distribution of seroprevalence of Salmonella agglutinins is shown in table 2. Data obtained in the study showed that in cattle, young (below 3 yrs) as well as adult cattle (>3 yrs) had almost similar sero–prevalence. Young buffaloes had higher sero–prevalence (15.3%) in comparison to that of adult buffaloes (8.4%). Similarly, young goats also had higher seroprevalence (5.2%) in comparison to that of adult (>1 yrs) goats (2.1%). The reason for this may be that young ones acquire the infection more easily due to their low immunity. In contrast, adult dogs had higher sero–prevalence (26.6%) in comparison to that of young dogs (22.3%). In agreement to our findings, Verma et al. (2008) have also reported high seroprevalence of Salmonella agglutinins in dogs with the increasing age, which might be either due to increase in susceptibility of dogs with age as in humans (Wray and Wray, 2000; Deborah et al., 2001) or repeated subclinical infection increased the antibodies detected by the test.
CONCLUSION
Present study has indicated the presence of Salmonella agglutinins in all the animal species tested. Dogs have shown the highest rate of seroprevalence of salmonellosis which further necessitates the control of disease among pets because of its zoonotic potential. Further studies employing large number of antigens and more specific tests would help in understanding the distribution pattern of Salmonella agglutinins in different regions of the country.
ACKNOWLEDGEMENTS
Authors are highly thankful to field veterinarians and other supporting staff for providing help in collection of samples; animal owners, who gave their permission for their animals to take part in this study.
REFERENCES
Basu S, Dewan ML and Suri JC (1975). Prevalence of Salmonella serotypes in India: a 16-year study. Bull. World Health Organ. 52(3): 331-336.
PMid:1084803 PMCid:PMC2366369
Chandra M, Singh BR, Shankar H, Agarwal M, Agarwal RK, Sharma G and Babu N (2007). Prevalence of Salmonella antibodies among goats slaughtered for chevon in Bareilly (Northern India). Prev. Vet. Med., 15 (1): 1-8.
http://dx.doi.org/10.1016/j.prevetmed.2007.01.008
PMid:17336413
Deborah H, Johan W, Vo AHo, Diep TS, Nguyen TC, Phan VB, Ha Vinh, Minh D, Christopher MP, Gordon D, Nicholas JW, Hien TT and Jeremy JF (2001). Serology of Typhoid fever in an Area of Endemicity and its Relevance to Diagnosis. J. Clin. Microbiol. 39(3): 1002-1007.
http://dx.doi.org/10.1128/JCM.39.3.1002-1007.2001
PMid:11230418 PMCid:PMC87864
Dhama K, Rajagunalan S, Chakraborty S, Verma AK, Kumar A and Tiwari R and Kapoor S (2013). Food-borne pathogens of Animal origin-Diagnosis, prevention and control and their zoonotic significance- A review. Pak. J. Biol. Sci. 16(20): 1076-1085.
http://dx.doi.org/10.3923/pjbs.2013.1076.1085
PMid:24506006
Kumar R, Verma AK, Kumar A, Srivastava M and Lal HP (2012a).Prevalence and antibiogram of Campylobacter infections in dogs of Mathura, India. Asian Journal of Animal and Veterinary Advances. 7(5): 734-740.
http://dx.doi.org/10.3923/ajava.2012.434.440
Kumar R, Verma AK, Kumar A, Srivastava M and Lal HP (2012b). Prevalence of Campylobacter spp. in dogs attending veterinary practices at Mathura, India and risk indicators associated with shedding. Asian Journal of Animal and Veterinary Advances. 7(8):754-760.
http://dx.doi.org/10.3923/ajava.2012.754.760
Muktaruzzaman M, Haider MG, Ahmed AKM, Alam KJ, Rahman MM, Khatun MB, Rahman MH and Hossain MM (2010). Validation and refinement of Salmonella pullorum(SP) colored antigen for diagnosis of Salmonella infections in the field. International Journal of Poultry Science. 9 (8): 801-808.
http://dx.doi.org/10.3923/ijps.2010.801.808
Niyogi SK, Dutta D, Bhattacharya MK and Bhattacharya CK (1999). Multi-drug resistant non-typhoidal Salmonella spp. associated with acute diarrheal disease. Indian J. Med. Res. 110:183-185.
PMid:10701296
Raichowdhuri AN, Agarwal P, Singh M and Anand BR (1983). Salmonella etiology of acute diarrhoea.J.Commun. Dis., 15: 8-13.
Singh S, Agarwal RK, Tiwari SC, Kumar K and Nambiar K (2010). Predominance of Paratyphi B var Java serotype among human and animals clinical isolates of Salmonella in North India. J. Vet.Pub. Hlth. 8(2): 73-81.
Snedecor GW and Cochran WG (1980). Statistical Methods. Oxford and IBH publishing Co. Calcutta.
Verma AK, Sinha DK and Singh BR (2007). Salmonella in apparently healthy dogs. Journal of Veterinary Public Health. 5(1):37-39.
Verma AK, Sinha DK and Singh BR (2008). Micro-agglutination test (MAT) based seroepidemiological study of salmonellosis in dogs. Journal of Immunology and Immunopathology. 10(1):29-35.
Verma AK, Sinha DK and Singh BR (2011a). Seroprevalence study on salmonellosis in apparently healthy dogs by enzyme linked immunosobent assay. Indian Journal of Animal Sciences. 81(1): 3-5.
Verma AK, Sinha DK and Singh BR (2011b). Detection of Salmonella from clinical samples of dogs by PCR. Indian Journal of Animal Sciences. 81(6): 552-555.
Wray C and Wray A (2000). Salmonella in domestic animals. C.A.B.I. (Publishers), London, United Kingdom.
http://dx.doi.org/10.1079/9780851992617.0000