Advances in Animal and Veterinary Sciences
Research Article
Clinical, Immunological and Epidemiological Studies of Nasopharyngeal Myiasis in Camels slaughtered in Al-Muthanna Province
Tareq Jaffer Al-Jindeel, Hussein Jabar Jasim*, Nawar Jasim Alsalih, Ali Mosa Rashid Al-Yasari
College of Veterinary Medicine, Al-Muthanna University, Iraq.
Abstract | The aim of this study was to investigate the clinical signs related to infected camels by C. titillator larvae and to determine prevalence and incidence of C.titillator larvae in the camels This study was conducted on 864 camels slaughtered at Al Muthanna abattoir during the period extended from 1st September 2015 to 30th August 2016. Fever, emaciation, loos of appetite loss, congestion of mucous membrane, enlargement of lymph nodes, nasal discharge, neurological signs, increase respiratory rate, frequent sneezing and snoring during breathing were the most common clinical signs of C. titillator infection. The numbers of infested camels by C. titillator larvae were 352(40.07%) out of 864. There were a relationship between the climatic conditions and the incidence of C. titillator larvae with a highest (89.02%) and lower (6.15%)percentages in January and July respectively. Additionally, the highest (70.1%) and the lowest(29.8%) infestation percentages were recorded in 4-7 years, and 8 months to 2 years old camels respectively. According to sex, infestation percentages were (56.6%) and (37.36%) for female and male respectively. This study was also showed variations in the mean concentrations of total protein (g/l), albumin, β-, γ-globulins between the infected and healthy camels. In conclusion, this study approved incidence of C. titillator infection in camelids with variation in the incidence that depend on the climate and age and sex of the animals recommend to do more studies in another provinces in Iraq to determine the epidemiological map of this parasites in camelids.
Keywords | Camels, Nasopharyngeal myiasis, Clinical, Epidemiology
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 12, 2018; Accepted | June 27, 2018; Published | July 25, 2018
*Correspondence | Hussein Jabar Jasim, College of Veterinary Medicine, Al-Muthanna University, Iraq; Email: husseinjasem2014@gmail.com
Citation | Al-Jindeel TJ, Jasim HJ. Alsalih NJ, Al-Yasari AMR (2018). Clinical, immunological and epidemiological studies of nasopharyngeal myiasis in camels slaughtered in al-muthanna province. Adv. Anim. Vet. Sci. 6(7): 299-305.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.7.299.305
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Al-Jundeel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Recently, the number of studies on camelids families in term of science and research has greatly increased. Camel is physiologically and anatomically adapted to survive harsh conditions and play an important role in human’s life how lived in desert and semi-desert and even in irrigate land. Camels like other domestics animals are also exposed to various pathogenic, infectious agents and disease (Borji et al., 2010). The herd growth and productivity of camels are restricted by various infectious agents such as parasites, which lead to mortality and high morbidity (Bekele, 2010).
There are different types of ectoparasites that can affect the health of camels such as; sarcoptic mange, tick, and fly infestations (Oryan et al.,2008). Nasopharyngeal myiasis is one of the most important problems of camels due to their responsibility for significant economic losses due to the presence of the larvae of Cephalopina titillator, which is a common obligate parasite of the Oestridea family that attacks only camels (Abd El-Rahman,2010). In Iraq, camels were firstly infested with myiasis in 1977 (Abdul-Hab and Al-Affass,1977).
The adult fly is widely distributed in areas where camels are found (Hussein et al., 1982; Higgins, 1985). During part of its life cycle, the female fly darts towards the nostrils and deposits its larvae directly into the nasal cavity. The larval stages are sometimes known as `al-naghaf’ in Arabic, loosely translatable as’ nose worm’. The larvae crawl up to the nasopharyngeal and sometimes reach the paranasal sinuses and molt twice while attached to the nasopharyngeal and paranasal mucous membranes and remain attached to the mucous membrane of these organs for up to 11 months. During this time, they feed and cause extensive irritation and tissue damage (Bekele, 2001; Shakerian et al. 2011).Nasopharyngeal myiasis of camels causes severe economic losses such as; reduction of milk production, destroy host tissues and decrease body weight, fertility. Furthermore, myiasis has the ability to reduce host physiological functions, show difficulty in breathing, sneeze, extensive irritation, and tissue damage that has been reported in infested camels (Otranto, 2001; Razi Jalali et al., 2016).The severity of clinical signs depends on the damage caused by migrating larvae (Otranto, 2001). In acute cases, the infected camels may die from meningitis caused by secondary bacterial or viral infections (Musa et al., 1989).In addition, (Morsy et al., 1998) mentioned that the mechanical damages such as penetrating the ethmoid bone by the larvae may assist in the introduction of bacteria and viruses to the cerebrospinal canal.
Although considerable numbers of camels are reared in semi-arid areas of Iraq and they have important role in livelihood of people, very few studies have been done on clinical and epidemiological aspects of parasitic infestations, especially myiasis as a serious threat for camel health. The aim of this study was to determine the prevalence rate and the influence of seasonal variation, age and gender on the prevalence of the infestation. Moreover, the clinical signs associated with C. titillator larvae infection in camels are also described. The results of this study may provide a rationale starting point for treatment planning and controlling measurements against the fly and the larvae and may even shed the light on some aspects of the life cycle pattern and ecology of the parasite.
MATERIALS AND METHODS
Study Area and Animals
This study was carried out randomly on 864 camels slaughtered from the slaughterhouse of Alkider, Rumathia and Samawah in Muthanna province, south of Iraq during the period from September 2015 to August 2016.The number of camels slaughtered varied from 4 to 6 a day. The slaughterhouse was visited three days a week. The examined camels were arranged into three age groups; 8monthes-2 years old, 2-4 years old and 4-7 years old. Samples were collected from slaughtered camels with different sexes about 546 male and 318 female. Their age was determined on the basis of dental formula. Furthermore, general clinical examination was performed on all camels in the slaughterhouse and includes general condition examination (hump structure), body temperature and external shape such as the nature of the camels’ lint and weaknesses, respiratory rate, heart rate and examination of superficial lymph node. The signs of Myiasis were observed and recorded.
Samples Collection and Diagnosis
After slaughtering, the heads of the slaughtered infested camels were separated from the rest of the body. The head was split longitudinally to expose the different regions of nasal cavity, nasopharyngeal area, frontal sinuses and turbinate bones. Then, careful gross examination was performed to detect the presence of the first, second and third instars of Cephalopina titillater larvae and the possible gross abnormalities accompanied with the presence of the larval infestation. The cross damage to the site of attachment was describe and recorded. The recovered larvae from each camel were carefully collected, washed in physiological saline solution (NaCl 0.9%) and preserved in a separate container for each carcass with 70% alcohol. All samples were labeled (characteristics include sex and age of the animal and history) and transferred to parasitology laboratory of College of Veterinary Medicine/Al- Muthanna University. Subsequently, diagnosis of Cephalopina titillater larvae was done according to specification of posterior spiracles as described by (Zumpt,1965).
This study was conducted on 30 samples collected from infected and control camels with age range 8 month-7 years,10 samples from males 10 from females and 10 of apparently healthy camels . Blood samples (5 ml) for the analyses were collected from the jugular vein by vacationer tubes without anticoagulant. To evaluate total serum protein concentration and protein fractions, the blood serum was separated by centrifugation at 2,500 rpm for 10 min ,Serum protein fractions were separated using electrophoresis on cellulose acetate plate in barbital buffer, pH 8.6, at 180 V, 4 mA, for 15 min according to manufacturer’s instructions (Helena Biosciences, UK).
After separation, the protein bands were stained with Ponceau S for 10 min, then destained with 5% acetic acid for 2 min and dehydrated in methanol for 2 min, and cleared with clearing solution (30% glacial acetic acid, 70% absolute methanol, and 4% clear aid) for 10 min, Finally, after drying at 50-60°C for 15 min, the relative levels of separated proteins were scanned using a densitometer at 525 nm. Protein fractions were identified and quantified by a computer software (Zamani et al., 2012). Albumin to globulin (A/G) ratio was then calculated from the electrophoretic scan. Data were analyzed using SAS software package (ver 9.1.3). The normal distribution of the data was evaluated using Kolmogorov and Smirnov test. A two-sample t-test was used to compare the means between groups, and a P≤0.05 was considered to be significant.
RESULTS
The total numbers of clinically examined camels are 864 of various age and sex. Clinical examinations of camels subjected to the study were showed a varied clinical signs as revealed in Table 1 In this table, the most clinical signs were observed in infected camels include frequent sneezing, snoring during breathing and congestion of mucous membranes in 38.7%, 36.6 % and 35.9 % respectively. While the less common signs were observed in infected camels are the enlargements of lymph node, increase heart rate and neurological signs (head shaking and trying to rubbing the nose of other animals or walls) in 1.8%,9.2% and 14% respectively. Additionally, the current study revealed the three phases of larvae of C. titillator of camels infested (Figure 1). The larval phase of C. titillator is mainly passed in the third stage. The first larvae stage (L1) is only about 2-4mm in length and up to 15 mm in the second stage. While, the mature white or grey third-stage larvae grow up to 15-23 mm (Figure 1). The gross examination of carcass of C. titillator infested camels after slaughtering was restricted to the nasal cavity, nasopharyngeal area, frontal sinuses and turbinate bones. The present study revealed that the most larvae were found and attached to the mucosa of the Nasopharynx, while a rare were found in the nasal cavity. Larvae were also observed in the frontal sinuses and turbinate bones. The nasal cavities of C. titillator infested camel were congested and obstructed by thick, dark-colored mucus, sever inflammation and degeneration changes., Some larvae remain active and moving nearly freely, but others larvae were attached firmly to the pharyngeal mucosa by their hooks and when removed, firm reddish nodules marked the sites of attachment. Whereas, the mucous membrane of nasopharyngeal region of infested camels by myiasis was congested, swollen, hemorrhagic, edematous with abundant mucous secretion. Furthermore, many nodules were observed in other parts of the nasopharyngeal area and these nodules were hard in consistency and contained calcareous material at sites of larvae attachment. The damage of C. titillator infested camel greatly depends on the number of migratory larvae (Figures 2 and 3).
The results of epidemiological study revealed that only 352 out of 864 examined camels were infected by C. titillator larvae during the study period from September 2015 to August 2016at infestation rate of (40.07%). As is revealed in (Table 2), the percentage of infestation with C. titillator larvae was associated with specific seasons. The examination showed highest percentage of infestation with C. titillator larvae which occurs in January 89.02%, followed by February68.35%. Whilst, the lower percentage of infestation with C. titillator larvae occurs in July 6.15% followed by June 9.45 with significant differences (P<0.01) (Figure 4). Table 3 showed the prevalence rates and differences among the different age groups and sexes. In this table, the highest percentage of infestation with C. titillator larvae was recorded in females in 56.6%. Whereas, the lower percentage of infestation with C. titillator larvae were recorded in male (37.36%) and showed significant differences (P<0.01) (Figure 5). Furthermore, this table showed the highest percentage of infestation with C. titillator larvae that occurs in age group between 4 to 7 years in 65.61%, followed by age group between 2 to 4 years in 35.58%.While the present study showed that lower percentage of infestation were recorded in camels with age group between 8 months to 2 years old in 22.95% (P<0.0001).
Table 1: Show Important Clinical Signs Related to Infected Camels by C. titillator larvae.
|
Percentage of infection % |
Numbers of animals |
Clinical singes |
|
33.5 |
290 |
Emaciation |
|
18.28 |
158 |
Fever |
|
25.46 |
220 |
Loss of appetite |
|
35.9 |
311 |
Congestion of mucous membranes |
|
35.3 |
305 |
Nasal discharge (Sticky) |
|
23.14 |
200 |
Increase Respiratory rate |
|
9.2 |
80 |
Increase heart rate |
|
1.8 |
16 |
Enlargement of lymph node |
|
14 |
121 |
Neurological signs (shaking of the head) |
|
38.7 |
335 |
Frequent sneezing |
|
36.6 |
317 |
Snoring during breathing |
Cellulose acetate electrophoresis of serum proteins revealed five fractions: albumin, two α-globulins (α1 and α2), β-globulin and γ-globulin concentrations (mean ± SD) in both sexes (female and male) 40 infected and 10 healthy camels. Electrophoretic patterns for comparison are shown in Table 4 and 5.
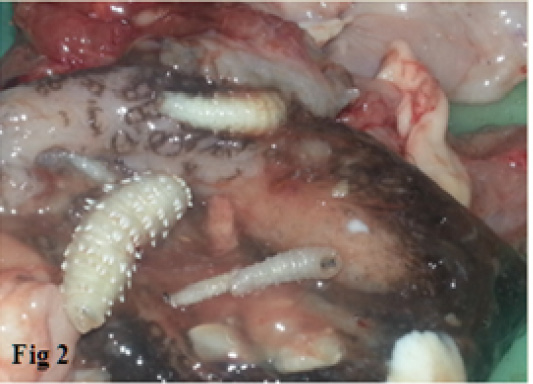
Figure 2: The nasopharyngeal region of infested camels by C. titillator showing Congested (dark color), Hemorrhagic, Swollen and Edematous mucous
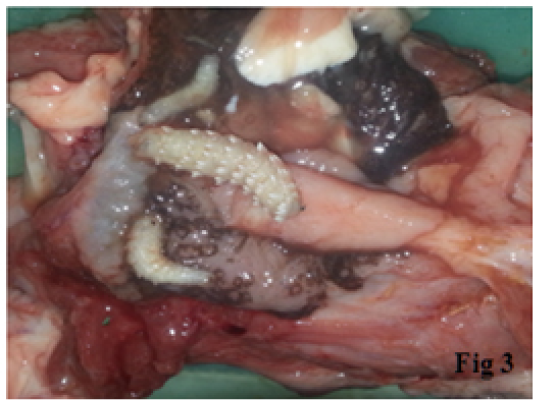
Figure 3: The nasopharyngeal region of infested camels by C. titillator showing Congested (dark color), Hemorrhagic, Swollen and Edematous mucous.
Table 2: Show the percentage of infested camels by C. titillator larvae according to months of years.
|
Months of year |
No. of camels examined |
No of infested camels |
Percentage of infestation % |
|
September |
66 |
21 |
31.81 |
|
October |
70 |
29 |
41.42 |
|
November |
78 |
40 |
51.28 |
|
December 2015 |
74 |
47 |
63.51 |
|
January 2016 |
82 |
73 |
89.02 |
|
February |
79 |
54 |
68.35 |
|
March |
72 |
41 |
56.94 |
|
April |
65 |
16 |
24.61 |
|
May |
70 |
11 |
15.71 |
|
June |
74 |
7 |
9.45 |
|
July |
65 |
4 |
6.15 |
|
August |
69 |
9 |
13.04 |
|
Total |
864 |
352 |
40.07 |
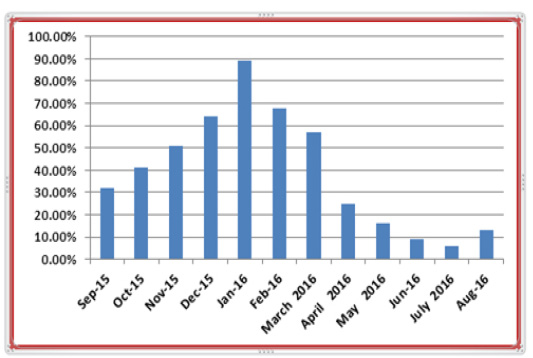
Figure 4: Show the percentage of infested camels by C. titillator larvae according to months of years.

Figure 5: Show the percentage of infested camels by C. titillator larvaebased on age (year) and sex.
Serum concentrations (mean ± SD) of total protein (TP) and protein fractions in apparently healthy camel (n=10) camels. The mean concentrations of total protein (g/l). Serum total protein concentration was significantly higher (p<0.01) in infected compared to healthy camels. The concentrations of albumin (g/l) in infected were 25.55±2.40 and healthy camels 40±3.10, respectively. This difference between infected and healthy camels was statistically significant (p>0.05). The mean concentrations (g/l) of α1-, α2-, β-, γ-globulins were 7.33.±0.14, 9.69 ±0.30, 29.92 ±0.80, and 24.05 ±0.53, respectively in infected camels and 4.33 ±0.22, 7.69±0.30, 22.06 ±0.30 and 13.05 ±0.20respectively in healthy camels. The mean concentrations of γ-globulins were significantly higher (p<0.01) in infected camels compared to healthy camels.
The mean concentrations of α1-, α2-, β-, were significantly higher (p<0.01) in infected camels compared to healthy camels.
Table 3: Shows the percentage of infested camels by C. titillator larvae based on age (year) and sex
|
Age |
Age | Sex | |||||||||
| Male | Female | ||||||||||
| Inspected | Infected | Percentage % | Inspected | Infected | Percentage % | Inspected | Infected | Percentage % | |||
| 8 months-2 years | 244 | 56 | 22.95 | 140 | 25 | 17.8 | 104 | 31 | 29.8 | ||
| 2-4 years | 399 | 142 | 35.58 | 282 | 93 | 32.9 | 117 | 49 | 41.8 | ||
| 4-7 years | 221 | 154 | 65.61 | 124 | 86 | 60.5 | 97 | 68 | 70.1 | ||
| Total | 864 | 352 | 40.74 | 546 | 204 | 37.36 | 318 | 148 | 56.6 | ||
Table 4: Show serum protein electrophoresis concentration measured to Infected Camels by C. titillator larvae.
| Index | Band | Ref. Area % | Conc. g/Lmean ± SD | Range g/L |
| 1 | Albumin | 25.55% | 25.00 ±2.40 | 35.00-50.00 |
| 2 | Alpha 1 | 7.56% | 7.33 ±0.14 | 1.00-4.00 |
| 3 | Alpha2 | 10.12% | 9.69 ±0.30 | 6.00-12.00 |
| 4 | Beta | 31.25% | 29.92 ±0.80 | 6.00-12.00 |
| 5 | Gamma | 25.12% | 24.05 ±0.53 | 7.00-15.55 |
| Total | 96.00 | |||
| Ratio | 0.28 |
Table 5: Show serum protein electrophoresis concentration measured to healthy Camels by C. titillator larvae.
| Index | Band | Ref. Area % | Conc. g/Lmean ± SD | Range g/L |
| 1 | Albumin | 45.55 % | 40.00 ±0.22 | 35.00-50.00 |
| 2 | Alpha 1 | 3.56% | 4.33±0.30 | 1.00-4.00 |
| 3 | Alpha2 | 8.12% | 7.69 ±0.30 | 6.00-12.00 |
| 4 | Beta | 10.25% | 22.6 ±0.20 | 6.00-12.00 |
| 5 | Gamma | 12.12% | 13.55 ±0.20 | 7.00-15.55 |
| Total | 87.97 | |||
| Ratio | 0.28 |
DISCUSSION
Nasopharyngeal myiasis, Cephalopina titillator larvae is one of the most non-ignorable problems of the livestock industry in many camel-producing areas of the world due to their responsibilities for significant economic loss to the camel industry. The results of the present study revealed that the local camels were infected with C. titillator larvae and this fact confirms that the Myiasis is widespread in areas where camels are found in Iraq.
There are few studies that have described the clinical signs in camels infested by C. titillator larvae. The present study revealed that C. titillator larvae infected camels showed similar clinical signs and came in line with (Hussein et al., 1982, Atiyah et al., 2011). Moreover, the results showed that the camels infected by C. titillator larvae clinically showed fever, emaciation, appetite loss, congestion of mucous membrane, enlargement of lymph nodes, nasal discharge, neurological signs, difficulty in breathing, frequent sneezing and snoring during breathing, all these signs were agreed with those reported (Zumpt, 1965; Otranto, 2001).The increase in body temperature occurs due to the stimulation of thermoregulatory center in the hypothalamus or result from hyperthermia, this stimulation occurs because of the release of endogenous pyrogens due to cellular lysis during the infestation (Zumpt, 1965). Furthermore, infestation with C. titillator larvae led to lose their appetite, could be attributed to present fever (Zumpt, 1965; Radstitis, 2000). Also, In heavy infection, the breathing in the camels Infested by C. titillator larvae is greatly impaired, frequent sneeze and snort during breating was explained as a result to blockage of the nasopharynx by larvae and/or muco-fibrinous secretions (Zumpt, 1965; Hussein et al., 1982).The congestion of mucous membranes was showed on conjunctivae and 3rd eye lid during the examination refer to hemostasis disturbances which may occur due to the thrombocytopenia and the disturbance of other clotting factors the as a result of increasing in the clotting time (Smith,1996). While, the other clinical signs such as; neurological signs and irritations may occur due to the larvae may reach the cranial cavity causing meningitis or some of the larvae penetrating the ethmoid bone may assist in the introduction of bacteria and viruses to the cerebrospinal canal (Hussein et al., 1982).
The overall rate of infestation among 864 examined camel heads was 40.7%.These results were agreed with (Abul-Hab, et al., 1977; Atiyah et al., 2011). This study showed that the highest percentage of infection with C. titillator larvae was occurred in Winter (January and February) with prevalence of infection 89.02%% and 68.35 % respectively. Whereas, lower percentage of infection with C. titillator larvae that were occurs in Summer, July followed by with prevalence of infection 6.15 % and 9.45 %respectively. These results were consistent with several previous studies which show that climate plays an important role in the prevalence of C. titillator larvae in the camels in different places of Iraq by Atiyah et al. (2011) in Al-Qadissiya province 42.43% and Abul-Hab and Al-Affass, (1977) in central Iraq was 46.7%. Also agreement with studies in other parts of the world conducted by Alahmed, (2002) in Saudi Arabia41%; Ramadan, (1997) in Egypt 37%; Al-Ani and Zuhair, (2016), Jordan 46.39. In our study, the results revealed that infestation rate with C. titillator larvae in cold weather were higher than it was in the warm season. Moreover, all three stages of larvae were found in each month of the year. This indicates that the flies may be found in all seasons, but in various level of abundance. In comparison with our results, previous studies have shown that camel bot fly is more prevalent in cold season (Fatani and Hilali, 1994; Oryan et al., 2008; Atiyah et al., 2011). The flies hatch out mainly during the rainy and moist season but infection during other times of the year could not be excluded. According to the life cycle, it seems the small size of the first stage larvae that may be overlooked can be the reason for the low prevalence in warm seasons (Fatani and Hilali, 1994). Perhaps, the larvae migrate from their predilection site towards the nasal passage during the warm dry season ready to be expelled and pupate in the ground (Razi Jalaliet al., 2016).
Furthermore, the present study showed that level of infestation with C. titillator larvae in females (56.6%) is higher than males 37.36%, these results indicate that the sex is a significant factor that affecting the prevalence of infestation with C. titillator larvae and agreed with several studies (Bekele, 2001; Atiyah et al., 2011), and disagreement with (Oryan et al., 2008) who reported that the percentage of infection is occurred in males 65 % is higher than in females 45.6%. The different susceptibility of C. titillator larvae between males and females may be due to the levels of sex hormones (Roberts et al., 2001; Atiyah et al., 2011). The breeding system may play an important role in the exposure variation of males and females because of most of the females used for the purpose of pregnancy and reproduction which could decrease resistance in females besides the lactation period, which are associated with hormonal and immunological changes. Whereas most of the males used for hard work and racing in many countries (Bekele, 2001; Kassa, 1995; Bassiony et al., 2005).
In addition, the present study showed that the high percentage of infection with C. titillator larvae occurs in age group 4- 7 years in 65.61%. Whereas, lower percentage of infection is occurred in age group 8 months to 2 years old in 22.95%.The results were in agreement with many previous studies (Abul-Hab, et al., 1977; Bekele, 2001; Oryan,et al., 2008).The difference in the distribution of infection with C. titillator larvae among age groups of study are explained by the camels of the group less than 2 years old were younger than a year and it is possible that they were born after the active season of the flies and were not exposed to infestation up to that time (Oryan,et al., 2008). Furthermore, the continuous exposure of these camels to the adult fly led to make the old camels more susceptible to be infested with C. titillator larvae than younger camels. All of that resulted in slowing down its movement and then their inability to put the first larvae that is newly developed by the adult fly through Sneezingor because of immunosuppression caused by progressive age (Atiyah et al., 2011).
Serum protein electrophoresis test has allowed specificity in the diagnosis, measuring the normal serum protein electrophoresis patterns in all domestic animals and the correct interpretation of their results is very useful for the clinician in diagnosing healthy and infected animals (Lutz et al., 2009).
The results of our study indicate that levels of α1-globulins, α2-globulins, β-globulins and γ-globulins were statistically significant increase in camels suffering from parasitic infection comprised to healthy.
The results were in agreement with many studies (Alberghina et al., 2010, Piccione et al., 2011; Tóthová et al., 2013). Serum protein electrophoresis standard method for fractionation and quantification of serum proteins is electrophoresis in clinical biochemistry.
Conclusion
Camels play an important role in the epidemiology of parasitic diseases under the three aspects of animal health, transmission to other livestock and zoonosis. Parasitic infections of camels may cause reduced milk and meat production, impaired fertility and decreased calving rates. They may also lower the working efficiency or even result in death and consequently high economic losses. The present work reflects the current state of knowledge on the parasitic fauna of camels in clinical, immunological and epidemiological studies on the parasites of camels in al-muthanna province. The serum protein electrophoresis and determination of absolute values of serum protein fractions in dromedary camels by cellulose acetate electrophoresis is very useful for clinicians to diagnose and evaluate various pathological conditions. The results presented in this study showed a significant effect of parasitic infection on the concentrations of some of the serum protein fractions in camels.
Acknowledgements
The authors are thankful to manager of veterinary hospital, the doctors and the workers of the slaughterhouse of Al-kider, Al-Rumathia and Al-Samawah in Muthanna province for their help during the collection of samples and many thanks to staff of departments of veterinary medicine and parasitology, college of veterinary medicine, Al muthanna university – Iraq for their support and providing the facilities during the samples processing.
COnflict of interest
The authors have not declared any conflict of interests.
Authors Contribution
All authors contributed equally.
References






