Advances in Animal and Veterinary Sciences
Research Article
Overview of New Concepts in Induce Ovulation Triggers in Dromedary Camels
Hella Jawad Al-Fatlawy*, Falah Hassan Baiee
Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Kufa, Iraq.
Abstract | The seminal plasma consists of several components that stimulate the ovulation process. The great quantity of an ovulation-inducing factor (OIF) in seminal plasma has wide implication questions about identification, sources, mechanism of action, role among species and clinical applications in the infertility. The purpose of the current review is to focus on the current understanding of physiological and biochemical properties of seminal plasma in camelids. llamas and alpaca seminal plasma was used as agent of induced and spontaneous ovulators. Column chromatography was used to identify the ovulation-inducing factor as part of seminal plasma that stimulating hormone secretion (LH) and ovulation in llamas. OIF is β-NGF that is highly conserved. An endocrine route of action of NGF explains a previously unknown pathway for the direct influence of the male on the hypothalamo–pituitary–gonadal axis of the inseminated female.
Keywords | Ovulation inducing-factor (OIF), Seminal plasma, Ovulation, Gonadotropins, Neurotrophins, NGF
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 12, 2018; Accepted | June 27, 2018; Published | July 25, 2018
*Correspondence | Hella Jawad Al-Fatlawy, Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Kufa, Iraq; Email: halaj.kadhim@uokufa.edu.iq
Citation | Al-Fatlawy HJ, Baiee FH (2018). Overview of new concepts in induce ovulation triggers in dromedary camels. Adv. Anim. Vet. Sci. 6(7): 292-298.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.7.292.298
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Al-Fatlawy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Hypothalamuses canpulsatile release the GnRH into the hypophyseal portal system with subsequent release of LH from the anterior pituitary into systemic circulation for inducing ovulation in mammals. The broad classification of species as either spontaneous or induced ovulators is based on the type of stimulus responsible for eliciting GnRH release from the hypothalamus (Adam et al., 2005). In spontaneously ovulating species (such as human, sheep, cattle, horse and pigs), the releasing of GnRH from the hypothalamus is triggered in the absence of progesterone and systemic estradiol concentrations exceed a threshold. In the induced ovulators (such as rabbits, ferrets, cats and camelids) the releasing of GnRH is contingent upon copulatory stimuli; hence, the ovulation is not a regular cyclic event (Bakker and Baum, 2000; Adam et al., 2005). Since a classic 1970 Peruvian study, dogma has maintained that physical stimulation of the genitalia during copulation is the primary trigger for inducing ovulation in alpacas and llamas. It is inaccurate to apply the term “oestrous cycle” in camels due to the pattern of ovulation in these animals,in contrast in spontaneous ovulators such as the cow and the mare (Bravo et al., 1990; Bravo et al., 1991).
Camels are seasonally polyestrous animals with estrous cycle differed from that in other farm animals. The estrous cycle in camel is characterized by three phases lasting 24-28 days with absence of the luteal phase. These phases are including follicular growth, existence of mature follicles (estrous period) and follicular atresia. The estrous period is longer up to 8 days (Shalash, 1980; Shalash, 1987; Skidmore et al., 1996; Skidmore et al., 2009).
Ovulation in the camel as in cat and rabbit occurs normally after the coitus. In these animals the neuroendocrine reflex involving the initiation of luteinising hormone release is delayed until coitus occurs (Jöchle, 1975). Manual stimulation of the cervix for 15 minutes in the camel did not induce ovulation but cause only partial luteinisation of the Graafian follicle, ovulation occurs 32 to 40 hours after copulation under the influence of luteinising hormone (LH) (Musa & Abu Sineina, 1978b; Wilson, 1984; Yagil, 1985).
Ovulation Induced Factor (OIF): Discovery and Role
Semen consists of seminal plasma, which is act as a fluid medium for swimming of spermatozoa. Seminal plasma is a complex fluid portion and mediates the chemical function of the ejaculate. Its pH varies with species, and it is slightly acidic in bulls and rams and slightly alkaline in camelids (Mann,1964). Rete testis, epididymis, and accessory sex glands of the male reproductive tract considered as a source of biochemical components of SP (Mann and Lutwak-Mann,1981). Accessory glands known as seminal vesicle, prostate, and bulbo-urethral glands contribute most of the volume of the ejaculate. The seminal vesicle secretion constitutes the major portion of seminal plasma (in most ruminants except camelids, in which it is absent) at ejaculation (Badawy and Youssef, 1982; Metafora et al, 1989).
The new detection of OIF in Bactrian camels fundamentally unnoticed for 20 years, its first established in llamas and alpaca wherein the ovulatory effect of seminal plasma was startlingly clear. It appears thatOIF in seminal plasma is conserved among both induced and spontaneously ovulating species (Adams et al., 2005; Ratto et al., 2005).
Recent findings explain the additional role of seminal plasma as an inducer of ovulation. The first direct sign of an ovulation-inducing factor (OIF) in semen came from workers in China, who reported that ovulation occurred after intravaginal or intramuscular administration of Bactrian seminal plasma to female Bactrian camels (Adams et al., 2005, England et al., 1969, Fernandez-Baca et al., 1970). The existence of the supposed OIF has gained little scientific attention for 20 years, until it has been confirmed in a series of studies involving Llamas and Alpacas (Ratto et al., 2005). Many reported documents described many OIF functions of the seminal plasma of alpacas, llamas and koalas (induced ovulators). It is also acted as a potent stimulator of LH secretion, and has a dose-dependent effect on ovulation rate and CL form and function. Moreover, it acts via a systemic rather than a local pathway, at physiologically relevant doses (Ratto et al., 2005; Chen et al., 1985; Sokol et al., 1985; Izumi et al., 1985; Paolicchi et al 1999).
Many observations revealed that the ovulation occurs after intravaginal and intramuscular administration of bacterian seminal plasma to female bacterian camels. The OIF in semen was recorded its existence for the first time in seminal plasma of llama and alpaca (Chen et al., 1985; Xu et al., 1985).
The OIF of camelids act as induced ovulators. It is an effective stimulator of luteinizing hormone (LH) secretion and it effects on ovulation, forming and function of the corpus luteum and acts via a systemic rather than a local pathwayat physiologically relevant doses (Ratto et al., 2006; Fernandez-Baca et al., 1970; Jöchle, 1975; Chen et al., 1985). Although the discovery of OIF in seminal plasma of species and categorized as induced ovulators (camel , cat and rabbit), the recent studies support the hypothesis that the OIF in seminal plasma isolated from other species which considered as spontaneous ovulators (cattle horse) (Bogle et al., 2011;Tanco et al., 2012, Ratto et al., 2011).
In llama, OIF of seminal plasmais a protein molecule that has a molecular mass of 30 kd. Despite, there are progressive results on the identification of OIF in SP, but its origin is not clear (Ratto et al., 2010). Pan et al. (2001) proposed that the synthesis OIF might be from hypothalamus or pituitary glandbecause they could not observe ovulation in female camels after vaginal deposition of AG fluids.
Comparative Approaches of the Oif Effects Versus Gnrh Effects
Many studies indicated that OIF and GnRH are two different molecules because OIF effect is more important than that of GnRH and their effect on pituitary LH release differently. The effect of OIF appears similar to that of GnRH used for the regulation of reproduction in camelids (Tibary and Anouassi, 1997; Skidmore, 2005). Adams et al. (2005) mentioned that intramuscular injection of seminal plasma in female llama showed a higher ovulation inducing rate (93%) than GnRH (83%). Moreover, higher level of plasma LH observed in seminal plasma treated female was faster but more extended as compared to the ones treated by GnRH, a significant increase in plasma LH concentration was seen 15 min after treatment with seminal plasma and 75 min after injection of GnRH. The maximum level was reached after 2 h with seminal plasma as compared to 1 h with GnRH, and as a final point, the decrease was delayed by 2.5 h in the seminal plasma treated group. In addition, the corpus luteum showed a greater diameter, regressed later, and produced more than two times progesterone in seminal plasma treated group as compared to the GnRH injected group. Although, the pattern of seminal induced LH flow is very similar to that described in response to natural mating (Adams et al., 2005; Bravo et al., 1990, Bravo et al., 1991). The progesterone secretion subsequent to ovulation is prolonged in OIF-treated group as compared to GnRH analog group (Fatnassi et al., 2017). Moreover, in female camel the luteotrophic activity of OIF is more important than that of GnRH. The OIF shows a dose–response effect on circulating concentration of LH and the incidence of ovulation in llamas and alpaca. However, GnRH purified OIF showed a dose–response relationship for the CL diameter and plasma progesterone concentrations. This indicates a possible luteotrophic effect of OIF, which is independent of the mechanisms involved by GnRH (Tanco et al., 2011; Ulloa-Leal et al., 2014; Stuart et al., 2015).
New Concept of OIF:NGF BETA
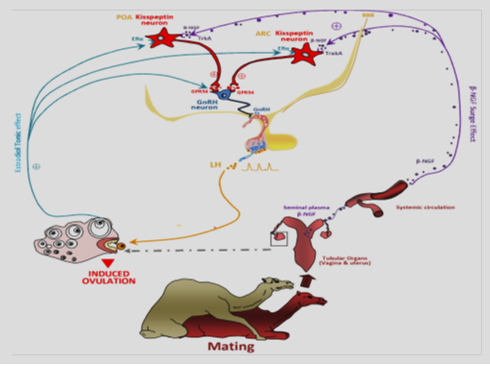
The intramuscular or intrauterine administration of seminal plasma in camelids was revealed inducing the pre-ovulatory luteinizing hormone (LH) flow and after ovulation and formation of corpus luteum. Ratto et al. (2011) has been identified OIF from SP as a neurotrophin, moreover the β subunit of nerve growth factor (β-NGF). β-NGF is well known as promoting neuron survival and growth, nonetheless in this case, it appears to induce ovulation through an endocrine mode of action. In fact, β-NGF may be absorbed through the endometrium to be conveyed, via the blood stream, to the central structures regulating the LH pre-ovulatory surge.
Ratto et al. (2005) suggested that OIF in seminal plasma induces ovulation by a systemic rather than a local route, although disagreement remains in one study in which the investigator observed ovulation after intravaginal deposition of alpaca semen in female alpacas and llamas (Sumar, 1994). Consequently, the recognized ovulation-inducing factor in SP is absolutely different from the native LH, human chorionic gonadotropin, pregnant mare serum gonadotropin, and prostaglandin-2a and possesses gonadotropin releasing hormone-like activity (Ratto et al., 2005). Surprisingly, its effects in the female were not identified earlier, given the profusion of NGF in seminal plasma. These observations are explaining the existence of the elaborate male accessory gland system as more than an evolutionary residues among species. The nerve growth factor is maintained significantly, and the identification of OIF as NGF shows recent discoveries of the effects of sperm plasma on the release of gonads and ovarian function in a variety of species. The purification product of β-NGF, from llama seminal plasma is high, and purification of large quantities allowed the discovery that NGF from the ejaculate has an important role in regulating gonadotropin release and ovarian function. The idea of an endocrine route of action of NGF on reproductive function is exclusive and explain a direct pathway for the influence of the male on the hypothalamo–pituitary–gonadal axis of the female (Figure 1) (Dissen et al., 1996; Barboni et al., 2002; Li, 2010; El Allali et al., 2017).
Determination of OIF as NGF in the seminal plasma llamas represents a unique sequence of the Camelidae and NGF derived from the only seminal plasma to be isolated, characterized, and fully sequenced in any species. Crystallographic study of purified OIF from seminal plasma provided the chance to determine the structure of natural NGF described previously only in the mouse, human, and cow. The purification of the mature and preforms of the protein in quantity from seminal plasma will permit in vivo study of the role of NGF in reproductive and neurologic health and disease (Abir et al., 2005; Julio-Pieper et al.,2006).
Isolation and Purification of OIF
The OIF in camel seminal plasma was isolated and purified
by several attempts using a combination of anion exchange and hydrophobic chromatography (Li and Zhao, 2004; Pan et al., 2001; Xilong and Zhao, 2004; Zhao et al., 2001), however, interpretation of the results is limited because of the lack of a validated bioassay to quantitatively test the effects of various fractions. Pan et al. (2001) suggested that OIF consists of a large folded complex of glycoprotein layers with bioactive forms composed of different molecules ranging from 16 to 54 kDav.
Effect and Routs of Administration of OIF
Series of experiments in alpacas and llamas were investigate to show the effect of rout of administration of OIF in seminal plasma on ovulation in female of the same species and to determine the route of action; i.e., local versus systemic (intramuscular and intrauterine administration)(Adams et al., 2005). In all experiments, OIF was given when a growingfollicle≥8 mm was detected and ovulatory capability existed. More than 4 separate experiments, intramuscular administration of seminal plasma (equivalent to<1/4 of an ejaculate) resulted in ovulation in 33/35 (94%) females compared to 0/35 (0%) given saline. However, intrauterine administration of seminal plasma resulted in ovulation17/44 (39%) females compared to 0/42 (0%) females given saline (Wabersk et al., 1995).
Dose Response and Mechanism of Action
The dose of purified OIF from llama seminal plasma required to stimulate an ovulatory response has been determined previously (Tanco et al., 2011) and it was physiologically acceptable in terms of the proportion present in a normal ejaculate. The female llamas were given a single intramuscular dose of 500 _g, 250 _g, 125 _g, or 60 _g of purified OIF (representative of the amount present in 1/25th to 1/200th of a normal ejaculate). A clear dose–response relationship was observed in circulating LH concentration, additionally the incidence of ovulation, maximum CL diameter, and day-to-day profiles ofCL diameter and plasma progesterone concentrations. All the experiments reach to the conclusion that seminal plasma OIF has a dose-dependent effect on ovulation, CL form and function, and that the biological effect of OIF is obvious at physiologically relevant doses. The native idea, ovulation in mammals involves pulsatiles ecretion of gonadotropin-releasing hormone (GnRH) from the medio-basal nuclei of the hypothalamus into the hypophy seal portal system, followed by the release of LH from the anterior pituitary into systemic circulation (Karsch, 1987; Johnstonn et al., 2004). The elevated circulating concentrations ofLH exist a flow of events within the mature follicle resulting in follicle wall rupture and evacuation of its fluid and cellular contents (Richards et al., 2002). While itis clear that the OIF in seminal plasma is effects on the ovulation by mediated through a surge release of LH into circulation. It is unapparent whether the site of action is at the level of the pituitary, hypothalamus, or both. Bogle et al., (2012)designed study to test the hypothesis that OIF stimulate LH secretion directly at the level of the pituitary. The cells from the anterior pituitary of llamas were cultured in vitro and LH concentration was measured in the culture medium after treatment. Treatment with OIF and GnRH induced more LH secretion than untreated controls, and LH concentrations were greater in wells treated with higher doses of OIF or GnRH compared to wells treated with alower dose. This is in agreement with the dose–depending effect of OIF observed in vivo (Tanco et al., 2011), and a previous study in which alpaca SP stimulated LH secretion from rat anterior pituitary cells in vitro (Paolicchi et al.,1999). Although these results do not keep out a possible effect of hypothalamus level, they confirmed that OIF has a direct effect on pituitary gonadotrophs independent of hypothalamic input GnRH (Silva et al., 2011).
Role of OIF Among Species
The OIF induce ovulation in induced ovulators animals and it is also influenced ovarian function in species considered to be spontaneous ovulators. OIF induced ovulation in a prepubertal mouse model (Xu et al., 1985), besides it altered ovarian follicular wave dynamics in cows (Zhao et al., 2001). The effect of seminal plasma in induction of ovulation is limited. In some earlier studies, it was documented that the sterile copulation with vasectomized males was related with improved LH secretion and a higher ovulation rate in spontaneous ovulators like cattle and sheep (Marion, 1950). Other authors also revealed that ovulation occurred after intravaginal or intramuscular/intrauterine administration (Chen et al., 1985; Pan et al., 1992) of Bactrian SP to female Bactrian camels. Lately, Adams et al., (2005) have documented the existence of ovulation-inducing factor (OIF) in seminal plasma of alpacas and llamas that could stimulate a surge in circulation. OIF is considered not to be species specific because bull seminal plasma can induce ovulation in she Bactrian camels (Chen et al., 1985), llama, and alpaca (Ratto et al., 2006). Though, the ovulation rate was lower with bull seminal plasma than with species-specific SP, the OIF might be a preserved molecule in seminal plasma among all mammals. Many studies supported hypothesis that OIF in SP is conserved among species, including those considered to be induced ovulators as (camel and cat) as well as spontaneous ovulators (bovine, equine and porcine) (Johnston et al., 2004; Ratto et al., 2006b; Bogle et al., 2011). OIF is based on the type of stimulus responsible for eliciting GnRH release from the hypothalamus (Bakker and Baum, 2000).In spontaneously ovulating species (human, sheep, goats, cattle, horse, pigs), the releasing of GnRH from the hypothalamus is triggered in the absence of progesterone and exceeding the systemic estradiol concentrations the certain threshold (Jaffe and Keys, 1974; Chenault et al.,1975; Kelly et al., 1988; Turzillo and Nett, 1999). Consequence, regular luteolysis is occurring that lead to development of one or more estrogen-producing follicles and a pre-ovulatory surge in circulating concentrations of LH at regular intervals. In induced ovulators (rabbits, ferrets, cats, camelids), the neural signals from copulatory stimulation trigger GnRH secretion from the hypothalamus that followed bythe preovulatory release of LH from the pituitary (Bakker and Baum, 2000). The existence of OIF has also recently been documented in horses and pigs SP (Bogle et al., 2011), but the incidence of ovulation was lower in llamas treated with seminal plasma from stallions and boars similar to bull seminal plasma. This observation is concluded that OIF in the seminal plasma of these species is in lower concentration or it is different and perhaps species-specific isoform. Interestingly, seminal plasma of rabbits (also an induced ovulator)induced ovulation in llamas, but not in rabbits (Silva et al., 2011a). Treatment of rabbit with seminal plasma was associated with a significant increase in the total number of antral follicles and hemorrhagic of the ovulatory follicles that detected at laparotomy (Silva et al., 2011a).
In conclusion, this review article focused on the ovulation-inducing factor (OIF) in llama and alpaca seminal plasma that is composed of a protein molecule and resistant to heat and enzymatic digestion with proteinase K. OIF has a molecular mass of approximately equal or higher than 30 kDa. The ovulation in mammalian females is classified into spontaneous and induced ovulators based on the mechanism stimulating ovulation. Ovulation in spontaneous species (human, sheep, cattle, horse, most rodents) occurs at standard intervals and depends upon the circulating estradiol. However, in induced ovulators (rabbits, ferrets, cats, and camelids), ovulation is associated with coitus. There is different factors lead to trigger ovulation, including auditory, visual, olfactory, and mechanic stimuli. Moreover, other studies have identified a biochemical component in the semen of induced ovulators responsible for the induction of ovulation and named accordingly ovulation-inducing factor (OIF). In camelids, intramuscular or intrauterine administration of seminal plasma was shown to induce the pre-ovulatory luteinizing hormone surge followed by ovulation and formation of corpus luteum. The OIF has been identified from seminal plasma as a neurotrophin, the β subunit of nerve growth factor (β-NGF). β-NGF is well known as promoting neuron survival and growth and it acts to induce ovulation through an endocrine mode of action.Indeed, β-NGF may be absorbed through the endometrium to be transfervia the blood stream and to the central structures regulating the LH pre-ovulatory surge.
Acknowledgements
The authors would like to thanks the University of Kufa /College of Veterinary Medicine for encouragement of their scientist.
Conflict of interest
There is no conflict of interest regarding the publication ofthis manuscript.
Authors Contribution
The authors contribute equally in this article.
References