Advances in Animal and Veterinary Sciences
Research Article
Evaluation of the Efficacy of a Polyherbal Formulation (Bovituss®) as a Supportive Treatment in Respiratory Infections of Bovines
Rajiv Walia1, Pranshu Sharma2, Kotagiri Ravikanth2, Bhaskar Ganguly2*
1Senior Veterinary Officer, Sub-Divisional Veterinary Hospital, Nalagarh, Himachal Pradesh; 2Clinical Research Unit, R&D Division, Ayurvet Limited, Baddi, Himachal Pradesh, India.
Abstract | The present trial was undertaken to evaluate the efficacy of a polyherbal formulation, Bovituss® (M/s Ayurvet Ltd., India), as a supportive treatment for alleviating the clinical signs of respiratory distress. Forty-two cows and she-buffaloes, aging four to nine years of different lactational stages suffering from respiratory tract infection were selected and divided into two equal groups viz. Group 1, receiving standard parenteral therapy with antibiotics and antipyretics, and Group 2, additionally receiving 30 mL Bovituss orally twice daily for a period of five days. The animals were examined on days 0, 3, and 5 of treatment. A significant reduction in the severity of respiratory signs was observed in Group 2. Significant improvement in feed intake was seen only in Group 2 and the overall clinical indices of both the groups calculated for all the three days revealed a significant improvement in Group 2.
Keywords | Bovituss, Bovine, Herbal, Respiratory distress, Respiratory infection
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | January 19, 2018; Accepted | April 18, 2018; Published | June 20, 2018
*Correspondence | Bhaskar Ganguly, Clinical Research Unit, R&D Division, Ayurvet Limited, Baddi, Himachal Pradesh, India; Email: clinical01@ayurvet.in
Citation | Walia R, Sharma P, Ravikanth K, Ganguly B (2018). Evaluation of the efficacy of a polyherbal formulation (bovituss®) as a supportive treatment in respiratory infections of bovines. Adv. Anim. Vet. Sci. 6(6): 252-257.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.6.252.257
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Walia et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Respiratory infections are common in livestock and form a major cause of economic loss to dairy entrepreneurs. The conventional management of respiratory infections requires the use of antimicrobials, for inhibiting the causative agent, and anti-inflammatory drugs, for suppressing the inflammation of the respiratory tract, thereby preventing loss of pulmonary function (Kariyawasam et al., 2007). Herbs have been used in folk medicine since many years and the use of herbal products as a therapeutic tool has been on the rise. Several natural compounds derived from herbs are known to significantly affect cellular mechanisms and evidence of their beneficial effects in inflammatory pulmonary conditions has been increasing (Slader et al., 2006). The present study was undertaken to evaluate the clinical efficacy of a polyherbal formulation, Bovituss® (M/s Ayurvet Ltd., India), as a supportive treatment in bovines suffering from respiratory infections.
MATERIALS AND METHODS
The field trial was conducted in bovines suffering with respiratory tract infections during October to December, 2017, in the Dhatwal area of district Hamirpur and Nalagarh sub-division of district Solan, Himachal Pradesh, India.
Forty-two cows and she-buffaloes, aging four to nine years and belonging to different lactation groups, were identified based on the clinical signs. The animals were randomly assigned to either of two treatment groups viz. Group 1, receiving standard parenteral therapy with antibiotics and non-steroidal anti-inflammatory drug (different agents as per case and different doses as per body weight), and Group 2, additionally receiving 30 mL Bovituss® orally twice daily for a period of five days.
Clinical examinations were performed on days 0, 3, and 5 of treatment. The respiratory index (Table 1a), based on
Table 1: Scheme for assigning Respiratory and Clinical Indices
|
a. Respiratory Indices |
|
| Parameter | Grading based on severity |
| Respiratory rate |
0 → 15-20/min 1 → 20-25/min 2 → 25-30/min 3 → >30/min |
| Nasal discharge |
0 → Small amount of normal serous discharge 1 → Small amount of unilateral cloudy discharge 2 → Bilateral, cloudy or excessive mucus discharge 3 → Copious bilateral muco-purulent discharge |
| Coughing |
0 → No coughing 1 → Mild cough with no exudate 2 → moderate cough with little or no exudate 3 → Severe coughing with copious exudate |
| Lung sounds (Intensity or severity of rales/ crackles) |
0 → Slight wheezes 1 → Scattered rales 2 → Wheezes, rales 3 → Severe rales/ crackles |
| Dyspnea |
0 → Good air movement 1 → Depressed air movement 2 → Diminished air movement with slight to moderate dyspnea 3 → High inspiratory or expiratory dyspnea |
|
b. Clinical Indices |
|
| Parameter | Grading based on severity |
| Fever |
0 → 99.5-102°F 1 → 102-103°F 2 → 103-104°F 3 → >104°F |
| Demeanor |
0 → Normal 1 → Mild restlessness 2→ Moderate lethargy 3 → Severe lethargy |
| Feed intake |
0 → Good 1 → Slightly reduced 2 → Moderately reduced 3 → Markedly reduced or anorectic |
respiratory rate, nasal discharge, coughing, lung sounds on auscultation and grade of dyspnea, and the clinical index (Table 1b), based on fever, demeanor, and feed intake, were compared across both groups. Grades were assigned on a scale of 0 to 3, 3 being the most severe. The group-wise data was subjected to two-way analysis of variance (ANOVA) for ascertaining statistical significance of the results.
RESULTS AND DISCUSSION
The animals were examined on days 0, 3, and 5 of treatment and all the clinical signs were graded for calculating parameter-wise indices per group. Animal-wise grades are shown in Table 2. The cumulative results are summarized for comparison in Figures 1, 2,3 and 4.
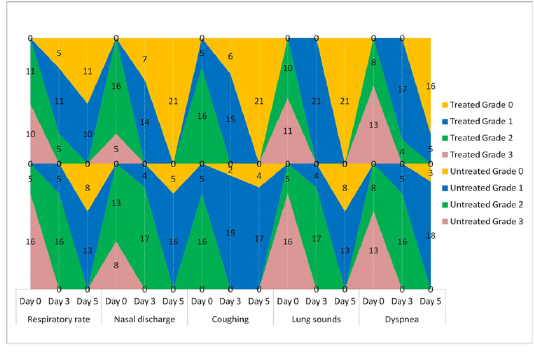
Figure 1: Frequency-distribution of animals according to grade of respiratory parameter. Values indicate the number of animals falling under a grade on the specified day of study.
Table 2: Animal-wise Respiratory and Clinical Indices
| Parameter | Group | Day | Clinical Respiratory Score | ||||||||||||||||||||
| Animal Number | |||||||||||||||||||||||
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI | XVII | XVIII | XIX | XX | XXI | |||
| Respiratory rate | 1 | 0 | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 |
| 3 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | ||
| 5 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
1 |
||
| 2 | 0 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 2 | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 3 | |
| 3 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | ||
| 5 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | ||
| Nasal discharge | 1 | 0 | 3 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 3 | 3 | 2 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 2 | 2 |
| 3 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | ||
| 5 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 2 | 0 | 2 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| 3 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Coughing | 1 | 0 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 |
| 3 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 5 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | ||
| 2 | 0 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | |
| 3 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Lung sounds | 1 | 0 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 2 | 3 |
| 3 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 |
2 |
||
| 5 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| 2 | 0 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 3 | 2 | 3 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Dyspnea | 1 | 0 | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 3 | 2 | 2 | 2 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 3 |
| 3 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | ||
| 5 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 2 | 0 | 3 | 2 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 3 | 2 | 2 | 3 | 2 | 3 | 2 | 3 | 3 | 3 | 3 | |
| 3 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | ||
| 5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| Fever | 1 | 0 | 3 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 3 | 3 | 3 | 3 | 2 | 2 |
| 3 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 0 | 2 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | |
| 3 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Demeanor | 1 | 0 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
2 |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 0 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Feed intake | 1 | 0 | 3 | 3 | 2 | 3 | 2 | 2 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | 3 | 3 | 2 | 3 |
| 3 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | ||
| 5 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| 2 | 0 | 3 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | 2 | 2 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 5 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
0 |
||
Individual parameters as well as aggregates of the average respiratory and clinical indices were compared on days 0, 3, and 5 to assess the efficacy of the polyherbal formulation. A significant reduction (p < 0.05) in the severity of respiratory signs was observed in Group 2 as evident from the lower respiratory index. Comparison of individual clinical indices revealed improvement in only the feed intake of Group 2, while rectal temperature and demeanor of the animals remained almost unchanged. The aggregate clinical indices also revealed a significant improvement in Group 2 (Table 3).
Table 3: Overall Respiratory and Clinical Indices
| Groups | Respiratory Index | Clinical Index | ||||
| Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 | |
| Group 1 | 12.33 | 8.05 | 3.67 | 6.86 | 3.1 | 0.81 |
| Group 2 | 11.62 | 4.47 | 0.72 | 6.52 | 2.81 | 0.48 |
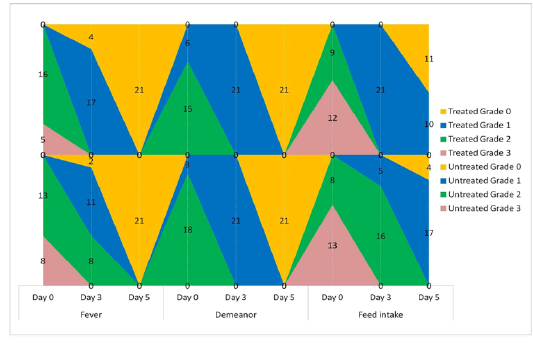
Figure 2: Frequency-distribution of animals according to grade of clinical parameter. Values indicate the number of animals falling under a grade on the specified day of study.
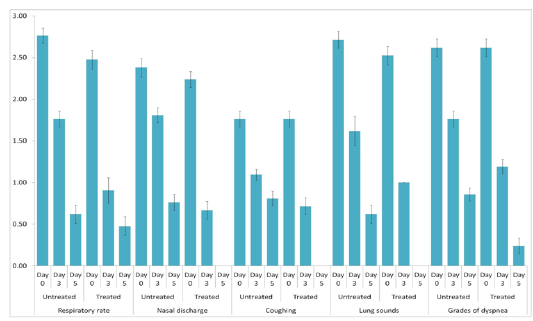
Figure 3: Respiratory index. Mean ± S.E. values of day-wise respiratory indices are shown for each group.
Bovituss is a scientific blend of various herbs and the clinical action produced by it is due to the varied action of the constituent herbs which act synergistically for better and faster recovery in respiratory tract infection. The key herbs present are Adhatoda vasica having antitussive (Jahan and Siddiqui, 2012) and anti-bacterial (Karthikeyan et al., 2014) properties; Ocimun sanctum having immunomodulatory (Mukherjee et al., 2005) and anti-inflammatory (Kothari and Sharma, 2012) properties; Hedychium spicatum showing bronchodilator, anti-inflammatory and analgesic activity (Ghildiyal et al., 2012); and Glycyrrhiza glabra showing anti-tussive (Jahan and Siddique, 2012), anti-inflammatory (Mirmala and Selvaraj, 2011), and anti-bacterial (Nitalikar et al., 2011) activity.
CONCLUSION
In the present study, forty-two cows and she-buffaloes suffering from respiratory infection were treated with a combination of parenteral antibiotic and non-steroidal anti-inflammatory drug with or without a polyherbal formulation, Bovituss®, as a supportive treatment. A significant reduction in nasal discharge, coughing, crackles/ rales, and dyspnea was observed in animals supplemented with the polyherbal formulation. Albeit comparable clinical indices, the animals additionally receiving the polyherbal preparation showed lower respiratory index, indicating greater relief from respiratory distress than those receiving conventional therapy alone.
Acknowledgements
The authors acknowledge M/s Ayurvet Limited for providing product samples and financial assistance for the study.
Conflict of interest
The authors declare no conflict of interest.
Authors Contribution
RW performed the study. PS revised the manuscript. KR planned and designed the study. BG performed data analysis & drafted the manuscript. All authors read and approved the final manuscript.
REFERENCES






