Advances in Animal and Veterinary Sciences
Research Article
Sero-epidemiology of Contagious Ecthyma Based on Detection of IgG Antibody in Selected Sheep and Goats Farms in Malaysia
Jamilu Abubakar Bala1,2, Krishnan Nair Balakrishnan1, Ashwaq Ahmad Abdullah5,6, Lim Cheng Yi1, Asinamai Athliamai Bitrus1, Yusuf Abba8, Isah Abubakar Aliyu2, Innocent Damudu Peter3,8, Idris Umar Hambali3,8, Ramlan Bin Mohamed7, Faez Firdaus Abdullah Jesse3,4, Abd Wahid Haron3, Mustapha M. Noordin1, Mohd Azmi Mohd Lila1**
1Virology unit, Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 2Microbiology Unit, Department of Medical Laboratory Science, Faculty of Allied Health Sciences, Bayero University Kano, Nigeria, P.M.B. 3011, Kano, Nigeria; 3Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 4Institute of Tropical Agriculture and Food security, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 5Institute of Bioscience, University Putra Malaysia, 43400 Serdang, Selangor Darul Ehsan, Malaysia; 6Department of Microbiology, Faculty of Applied Science, Taiz University Taiz Yemen; 7Institut Penyelidikan Haiwan, (IPH), Veterinary Research Institute, Ipoh, 59, Jalan Sultan Azlan Shah, 31400 Ipoh, Perak, Malaysia; 8Faculty of Veterinary Medicine, University of Maiduguri. P.M.B 1069 Maiduguri, Borno Nigeria.
Abstract | Contagious ecthyma (CE) is caused by parapoxvirus with the disease characterized by scabby lesion formation on the nostrils and mouth. Currently, there is paucity of information on its endemic status among small ruminants in Malaysia. Thus, this study was aimed to determine the status of CE among small ruminant in selected farms on clinical, molecular, epidemiologic, and virology aspects of the disease. IgG antibody detection was deployed as a yardstick for CE infection and it was assessed together with associated risk factors. A total of 180 serum samples from 90 sheep and 90 goats were obtained from 5 randomly selected farms. The samples were subjected to qualitative test for anti-CE IgG by Enzyme-Linked Immunosorbent Assay (ELISA) method. PCR was conducted on scab samples, targeting at immunogenic CE virus envelop protein (B2L) gene. Positive samples detected by serology and polymerase chain reaction (PCR) methods were subjected to viral isolation on chorio-allantoic-membrane (CAM) of embryonating-hen-eggs. It was revealed that prevalence rates of CE were 12.2% in sheep and 14.4% in goats. CE virus DNA was detected in the asymptomatic CE infection in sero-converted animals. CE virus was isolated in embryonating SPF eggs and identified. In sheep, there was a significant difference (p<0.05) in the prevalence rates among sexes, ages and type of farms. It was observed that poorly managed farms inclined to have higher prevalence rates. Asymptomatic CE in livestock is quite common and further investigation is warranted. In conclusion, CE infection is persistent in sheep and goat farms in Malaysia.
Keywords | Contagious ecthyma, Risk factor, Seroprevalence, IgG ELISA, PCR
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 27, 2017; Accepted | January 26, 2018; Published | May 31, 2018
*Correspondence | Mohd Azmi Mohd Lila, Virology unit, Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; Email: azmi@upm.edu.my; azmilila@gmail.com
Citation | Bala JA, Balakrishnan KN, Abdullah AA, Yi LC, Bitrus AA, Abba Y, Aliyu IA, Peter ID, Hambali IU, Mohamed RB, Jesse FFA, Haron AW, Noordin MM, and Mohd-Lila MA (2018). Sero-epidemiology of contagious ecthyma based on detection of IgG antibody in selected sheep and goats farms in Malaysia. Adv. Anim. Vet. Sci. 6(5): 219-226.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.5.219.226
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Bala et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Contagious ecthyma (CE) is an emerging, infectious and zoonotic viral disease of sheep and goat that is caused by CE virus belonging to the poxviridae family according to the classification of the international committee on taxonomy of virus (ICTV) (Tedla et al., 2018). The disease affects the skin and is characterized by the formation of a large benign tumor-like vascularized lesion that can be treated surgically or with antiretroviral drugs. (Abdullah et al., 2015a; Fleming et al., 2015; Sadiq et al., 2017; Tedla et al., 2018). It has a wide host range affecting sheep, goats and other wild animals such as alpacas, camels and reindeer. The morbidity of the disease can reach up to 100% and mortality due to secondary complications may reach 15% (Ramesh et al., 2008; Bora et al., 2012; Kumar et al., 2015). Marketability of sheep and goats for trading or slaughtering for meat purposes also declines due to the nasty dermatological lesion.The disease is endemic among small ruminants in Malaysia and has previously been reported (Sadiq et al., 2017). In a recent study, conducted by Jesse et al. (2018) in small ruminants, 36.7% and 7.8% seroprevalence of CE against IgM antibodies has been reported, respectively. CE has a zoonotic concern as humans can also be infected through open wounds and cuts (Buttner and Rziha, 2002). Lesions are caused through direct contact with infected material and usually develop locally at the hands. The occurrence is high among farm personnel during lambing, docking, shearing or slaughtering of positively infected animals (Nandi et al., 2011). According to Nandi et al. (2011) vaccination is the only choice to effectively control CE.Along with strict sanitation practices, vaccination reduced the disease to none by 1969 in Egypt (El-Dahaby et al.,1980). A study performed in Assam, North Eastern India to determine the seroprevalence of CE in goats (Bora et al., 2016) using traditional indirect Enzyme Linked Immunosorbent Assay (ELISA) showedseroprevalence of 76.6% after sampling n=231 goats. Sandwich ELISA was proven to bea better alternative for detection of antibodies against CE virus especially when the sample size is large. The methodis less cumbersome, cheap and the samples do not haveto be purified prior to analysis (Sum et al., 2017).
There have been reported CE outbreak in Malaysia for many years (Abdullah et al., 2015a; Sadiq et al., 2017; Jesse et al., 2018). Similar to diseases caused by other viruses, there is a need to revisit the probable differences in factors that influencing pathogenicity, immune responses and immunity (Azmi and Field, 1993) against orf virus. The limited use of standardized vaccine in Malaysia and Asian countries could be the main contributing enhancing factor (Bala et al., 2018; Jesse et al., 2018b). In part due to recent repeated outbreaks of CE in this region, changing epidemiological state to enzoonotic form, there is an urgent need for an extensive serological surveys on goat and sheep sample populations. In addition, there was an inadequate information on sero-epidemiology of past CE among small ruminants. The presence of IgG antibody in sero-converted animal shall serve as a good indicator for the presence of long-term CE infection and that is to discriminate from recently infected animals. This study aimed to determine sero-epidemiology of CE in small ruminant farms based on detection of IgG antibodies. Seropositive cases were further subjected to virus isolation by using specific-pathogen-free (SPF) eggs and molecular confirmation by polymerase chain reaction (PCR).The risk factors associated with CE infection was also determined.
MATERIALS AND METHODS
Ethical Statement
All experimental procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals approved by Institutional Animal Care and use Committee (IACUC) of Universiti Putra Malaysia, AnimalWelfare Act (2014) [AUP No.: FYP. 2016/FPV (32.50)]as legally required in Malaysia. All applicable institutional guidelines for the care and use of animals were followed.
Farm Data Collection
The study was conducted in small ruminant farms that were randomly selected from the state of Selangor, Malaysia. A total of eight (8) ruminant farms consisting of five (5) goat farms and three (3) sheep farms were sampled in this study. A total of n=180 small ruminants which consist of (n= 90) sheep selected from three farms (n=42 in Farm A, n=31 in Farm B and n=17 in Farm C. Similarly, n=90goats consisting of n=11 in farm A, n= 12 in farm B, n=26 in farm C, n= 20 in farm D and n=21 in farm E were enrolled in this study. The sampled animals consist of n=86 female and n= 4 male goats, n=77 females and n= 13 male sheep. In addition, the animals were categorized based on age as young and adult; a total of n=86 and n= 61 of the sampled animals were adult goat and sheep while, n= 4 and n=29 were young goat and sheep respectively.
Risk Factors
Well-structured questionnaire designed and administered to farmers and personnel working in the farm to ascertain the risk factors associated with the seroprevalence of CE against IgG antibodies. The risk factors considered in this study to include, age, sex, presence or absence of clinical signs, water sources, biosecurity and farm management.
Sample Collection
Blood samples were collected from a total of 180 animals (sheep; n=90; goat; n=90) randomly selected by convenience from small ruminant farms located in Selangor state. The animals were physically restrained, and three milliliters (3ml) of blood was obtained from each animal via jugular venipuncture into pre-labelled vacutainer tubes (5mL each into plain anticoagulant free tubes). The samples were then transported in an ice box to the laboratory for serological analysis.
Serum Extraction
Serum sample was harvested by centrifugation of blood at 3000 revolutions per minute (rpm) for 20 minutes (754 x g). The harvested serum were transferred into a fresh 1.5 mL microcentrifuge tube and kept at -20˚C until subjected to indirect Sandwich-ELISA test.
ELISA Test
The CE IgG ELISA was performed using sheep and goat microelisa strip plate (SunLong Biotech Co., LTD) according to the manufacturer’s instruction. Briefly, a total 50μL of negative and positive serum samples were added to the negative and positive control wells respectively. Additionally, 40μL of sample dilution buffer and 10μL of serum sample were added into the remaining 90 wells. Each sample was then mixed well with gentle shaking, sealed with a closure plate membrane and then incubated for 30 minutes at 37˚C. After incubation, the closure plate membrane was carefully removed and the reaction was washedwith a wash solution 5 times at interval of 30 seconds each. After 5 cycles of washing, 50μL of HRP-Conjugate reagent was added into each well except the blank control well.The incubation and washing procedure was once again repeated. After which 50μL each of Chromogen Solution A and Chromogen Solution B were added into each well, mixed with gentle shaking in a dark room and incubated for another 15 minutes at 37˚C. The reaction was stopped by the addition of 50 μL of stop solution into each well. A colour change from blue to yellow was observed in the well. The absorbance O.D (450nm) was read using a Microtiter Plate Reader within 15 minutes after adding stop solution. The effectiveness of the test kit was confirmed as the average value of positive control was more than 1.00. The cut off value was obtained by the following formula.
|
Cut off (CO) value = the average value of 2 negative controls + 0.15 |
From the OD readings, values less than the CO value were interpreted as negative for anti-CE antibodies; whereas OD values which weremore than the CO value were interpreted as positive.
Virus Isolation in Embyonating Specific Pathogen Free (SPF) Hen Eggs
Embryonating SPF hen eggs were inoculated in triplicates with virus suspension prepared from the processed scab. All eggs were first screened for their embryo survival by candling. After candling, air sac was marked, a needle was used to puncture the shell to create a hole on the 2-sides (dorsal and lateral) of an egg aseptically. A rubber bulb was used to pressure out the air from the egg’s air sac. This created a negative pressure that led to formation of a new airspace at the horizontal side of egg’s chorio-allantoic membrane (CAM) area. Scab suspension, at 0.1ml per inoculum, that may contain CE virus was inoculated through the hole. The holes were sealed with molten wax, and eggs were incubated at 37oC for 5-7 days. The eggs were checked on daily basis for determination of embryo mortality. The virus harvested, first by keeping the eggs at 4oC overnight. Eggs were wiped with 70% alcohol. A sterilized forceps was used to crack the egg and the shell was peel until CAM visible. CAM was gently removed to expose the embryo then placed in a sterile petri dish. Both CAM and embryo were checked for pock lesions and compared with control eggs. Pathology changes were observed, and the size of each embryo was compared with the control embryo. CAM and embryos were processed further in sterile pestle and mortar with an aid of small amount of sterile sand. At the ratio of 1:3 tissue to PBS suspension, virus sample suspension was prepared from CAM tissue and centrifuged at 2000rpm for 10minutes. The supernatant was separated and penicillin/streptomycin antibiotics was added to a final concentration of 1%. The supernatant was passed through 0.45um filter and aliquot in 1.5ml microfuge tubes. The aliquot was used as inocula for second passage of virus inoculation inembryonating SPF hen eggs. The virus isolated from positive samples was passaged five times in embryonating egg.
Molecular Confirmation of CE Virus by PCR
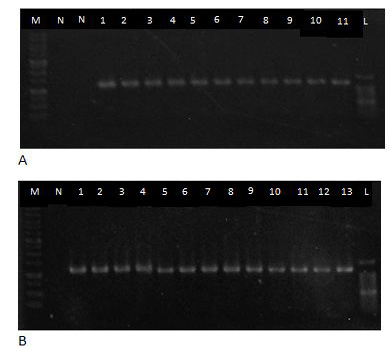
DNA extraction was carried out by using of viral nucleic acid extraction kit (Geneaid Biotech Ltd) (Abdullah et al., 2015a). DNA was extracted fromskin/scab tissue samples obtained from CE-seropositive animals was tested in PCR procedure. The procedure used a set of 2 primers, forward and reverse sequences, targeting at the most immunogenic envelop protein gene of the virus namely B2L gene (Abdullah et al., 2015a; Li et al., 2012; Inoshima et al., 2000). Forward primer sequence is 5-ATG TGG CCG TTC TCC TCT ATC-3 and reverse primer sequence is 5-TTA ATT TAT TGG CTT GCA G-3. PCR procedure was accomplished according to the manufacturer’s instruction (Novagen, Toyobo, Japan). Thermal cycler was programmed according to the following conditions; 95oC for 2 min as an initial activation step, followed by 35 cycles of 94oC for 20s; 50oC for 30s, 70oC for 20s, and one final cycle of 72oC for 2 min. PCR products were run in the agarose gel and electrophoresed at 100 V for 50 min. The gel was stained in Red Safe Nucleic Acid Staining Solution and DNA bands were viewed ingel documentation system (Abdullah et al., 2015a)
Data Analysis
Chi Square test statistics was used to analyze the data obtained in this study using IBM SPSS Statistical software version 22 to determine the seroprevalence of CE virus in small ruminants and the associated risk factors of the disease. Statistical significance was set at p< 0.05 at 95% confidence interval.
Table 1: Seroprevalence of Orf basedon age, sex and clinical signs among selected small ruminants farm in Selangor, Malaysia (n=180).
| Variables | No. positive | No. Negative | Prevalence (95% CI) |
| Farm (Goat) | |||
| A (n=11) | 1 | 10 | 9.1% |
| B (n=12) | 0 | 12 | 0.0% |
| C (n=26) | 6 | 20 | 23.1% |
| D(n=20) | 5 | 15 | 25% |
| E (n=21) | 1 | 20 | 4.76% |
| Overall prevalence (n=90) | 13 | 77 | 14.4% |
| Farm (Sheep) | |||
| A(n=42) | 2 | 40 | 4.76% |
| B (n=31) | 3 | 28 | 9.67% |
| C (n=17) | 6 | 11 | 35.3% |
| Overall prevalence (n=90) | 11 | 79 | 12.2% |
| Age | |||
| Adults (goat) n=86 | 13 | 73 | 15.1% |
| Young (goat) n=4 | 0 | 4 | 0.0% |
| Adult (sheep) n=61 | 4 | 57 | 6.6% |
| Young (sheep) n=29 | 7 | 22 | 24.1% |
| Sex | |||
| Goat (male) n=4 | 0 | 4 | 0.0% |
| Female n=86 | 13 | 73 | 15.1% |
| Sheep ( male) n=13 | 4 | 9 | 30.8% |
| Female n=77 | 7 | 70 | 9.1% |
| Clinical Signs | |||
| Goat (with C.S) n=3 | 1 | 2 | 33.3% |
| (without C.S) n=87 | 12 | 75 | 13.8% |
| Sheep (with C.S) n=5 | 1 | 4 | 20% |
| (without C.S) n=85 | 10 | 75 |
11.8% |
C.S =clinical signs, n=number sampled, No. = number, CI= confidence interval
RESULTS
Seroprevalence of CE Based on IgG
Based on the result obtained, the seroprevalence of CE based on IgG in sheep and goats were 12.2% (11/90) and 14.4% (13/90), respectively (Table 1). Farm level seroprevalence of CE virus showed that 9.1% (1/11) (farm A), 0.0% (0/12) (Farm B), 25% (5/20) (Farm C), 23.1% (6/26) (Farm D) and 4.76% (1/21) (Farm E) of all the goats sampled were seropositive for CE virus. In sheep, 4.76% (2/42) of all the animals in farm A were seropositive for CE, while 9.67% (3/31) and 35.5% (6/17) of all the sheep sampled from farm B and C seropositive to CE.
Risk Factors
The seroprevalence of CE based on sex showed that 30.8% (4/13) of male sheep were seropositive for IgG antibodies against CE than female sheep 9.1% (7/77). This showed that there was a statistically significant association between sex and the seroprevalence of CE in sheep (p<0.05). In goats, none of the male animals 0.0% (0/4) were seropositive for IgG antibody against CE, while 15.1% (7/77) of the female goats were seropositive for antibodies against CE virus (p>0.05); thus, indicating that there is no significant association between sex and seroprevalence of CEagainst IgG antibodies. In sheep, there was a statistically significant association between the seroprevalence of CE and age, 24.1% (7/29) of young sheep seropositive for IgG antibodies against CE than in adult sheep 6.6% (4/61) (p<0.05). In goats however, there was no statistically significant association between age and the seroprevalence of CE. Similarly, there was no statistically significant association between the presence or absence of clinical signs and the seroprevalence of CE against IgG antibodies both in sheep and goat (P>0.05). However, seroprevalence of CE in sheep with clinical signs of 20.0% (1/5) washigher than in sheep without clinical signs 11.8% (10/85). In goats, higher seropositivity was observed in goats with clinical signs 33.3% (1/3) than in goats without clinicalsigns 13.8% (12/87) (Table 1).
Isolation of CE Virus in SPF-Embyonating Hen Eggs
The progress of virus growth in SPF embryonating eggs was recorded as in Table 2. The typical appearance of CE virus lesion with thickening of CAM, edematous and hemorrhagic tissue is shown in Figure 1.
Detection of B2L Envelope Gene by PCR
DNA samples were subjected for PCR procedure and positive amplified bands typical for B2L gene were revealed as shown in Figure 2 and Table 3. Approximately 1092bp PCR amplified productsof B2L envelope gene of CE virus were produced in the procedure.
Table 2: Virus growth in SPF embryonating eggs
| Period | Characteristics observed |
| Day 1 | All embryos remain alive |
| Day 2 to 3 | All embryos remain alive |
| Day 5 to 7 | All embryos remain alive, darkening of membrane in eggs was noticed during candling |
|
Day 8 |
Thickening of CAM and presence of pock lesion- characteristics of CE virus infection |
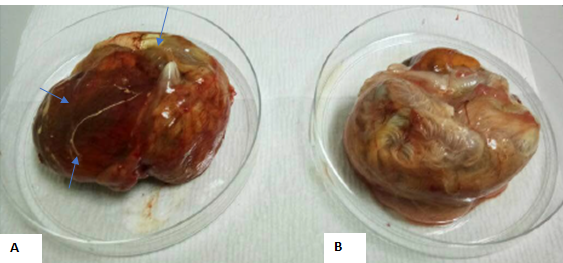
Figure 1: Exhibit A showed edematous lesions on the membrane, hemorrhages and thickening of CAM, while exhibit B showed an un-inoculated chick embryo with normal CAM.
Table 3: Prevalence of CE in small ruminants based onIgG Antibody and PCR confirmation
| Animal species | Total No. of samples | IgG | PCR detection confirmation (%) | |
| No. of Positive (%) | No. ofNegative (%) | |||
|
Sheep Goat Total |
90 90 180 |
11(12.2) 13(14.4) 24(13.3) |
79(87.8) 77(85.5) 156(86.7) |
11(100) 13(100) 24 |
| P Value | 0.050 | |||
DISCUSSION
The disease distribution according to the possible risk factors was evaluated to determine potential control measures. The occurrence of CE disease can be influenced by multiple factors such as age, breed, location of the farm and farm management. Meanwhile, serology and CE virus identification could be readily carried out by combining several useful assays such as ELISA, EM, FAT, AGID, and confirmed by PCR and viral isolation (Loh and Mohd-Azmi, 2009). This study used IgG detection assay, virus isolation and PCR technique to confirm CE infection and reveal probable association between prevalence rates based on gender, age and farm in related to population of sheep and goat.
The overall prevalence of CE determined based on IgGwas 14.4% in goats and 12.2% in sheep. This finding is in agreement with our recent study where goats had CE seroprevalence of 36.7%, while sheep had seroprevalence of 7.8% based on IgM detection (Jesse et al., 2018). Overall prevalence rates determined by this study were very much lower compared to that of 34.89% as reported by Goa et al. (2016) in China. However, this was determined by PCR procedure based on B2L gene of CE virus.
Goats are commonly moreprone to contract CE with high severe manifestation than their sheep counterpart (Guo et al., 2003). One of the major factor is goats commonly exhibit more aggression than sheep. Fighting among goats are very common especially in intensively managed production system (Orgeur et al., 1990). The aggression linked to higher testosterone levels as stated by Archer, (1991) putting them in a risk of fighting and getting injured. Meanwhile, CE virus can remain persistence in an environment under shade for long time (Popma et al., 2007; Sadiq et al., 2017; Fleming and Mercer, 2007). The horn causes a cut or abrasion leading to inoculation of an existing virus from the environment. This poses a risk of having previously infected animals in the farms.
The prevalence rate of CE determined in lamb was higher than in young goats. This concurs with a previous study by Nandi et al. (2011). Similarly, Onyango et al. (2014) documented a higher prevalence of 1.88% in ewes and 19.51% in lambs. Lambs have higher probability of contracting the disease compared to adults and this is attributed to the immune system of lambs that is still underdeveloped and it relies mostly on maternal antibody and innate immunity for protection against CE virus. Since vaccination against CE was not currently in practice among farms studied, both dams and lambs were not protected against CE infection and there is a high probability for lambs to get CE virus infection and developing clinical diseases.
There was no significance association between clinical signs and actual prevalence rate of CE in sheep for this study. The clinical signs are usually associated with recent infection (Abdullah et al., 2015a; Sadiq et al., 2017; Jesse et al., 2018). As goats may develop more severe lesions compared to sheep hence clinical signs become unreliable as the first line of diagnoses towards CE infection.
The prevalence rate measured in this study was based on detection of IgG antibody in sero-converted animals, a parameter for detection of past infection or exposure to CE virus. Clinical signs are more commonly seen in the first exposure as compared in secondary or subsequent infections. Primary immune response takes a longer time to reach a peak in order to establish immunity that enable infected animals to recover from infection. Similar to responses to many other viral infection, secondary immune response becomes effective within a shorter period of time and usually with higher magnitude of responses (Jesse et al., 2018; Bala et al., 2012).
The farms that did not practice adequate biosecurity showed the highest seroprevalence of CE infection and it also had a recent outbreak of CE infection. From the history, this farm practices importation and trading of sheep from unreliable sources and this can be the reason for recent outbreak in the farms. This agrees with our recent study, where detection of CEIgM was higher in farms practiced poor health heard program (Jesse et al., 2018a). This shows that there was poor Herd Health Programme (HHP) compliance by the farmer and in accord with the survey by Abdullah et al. (2015b), which stated that small ruminant farm personnel in Malaysia have inept knowledge and skills required for effective HHP. This particular farm was intensively managed hence there is the possibility of more contact between the animals, increasing the likelihood of contracting the disease. The economic losses associated with CE infection in intensively managed.
Orf virus was isolated from all suspected infected host and grown in CAM of SPF eggs despite failed attempts by other researches (Kumar et al., 2015; Bala et al., 2018; Jesse et al., 2018b). Detail studies on adaptation of orf virus in tissue culture and embryonating eggs would pave the way for further studies to elucidate orf virus biology, mechanisms and preparation of the virus for vaccines to control and eradicate the disease (Abdullah et al., 2015a; Sadiq et al., 2017; Bala et al., 2018). It would be rather intriguing to determine the capability of orf virus to grow in cell lines of the original host perhaps to affect cell growth in vitroas well (Vakhshiteh et al., 2013; Hani et al., 2010). Besides parapoxviruses, herpesvirus family also portraying similar characteristics to grow in cell lines Balakrishnan et al. (2017)
PCR and viral isolation were employed as confirmatory diagnosis for diseases. These assays has helped to distinguished orf virus from its likes such as FMD, sheep pox, blue tongue and bovine herpes virus which were all incriminated to cause similar diseases that are affected goat and sheep. All the serologically positive animals were confirmed by PCR test on the associated animal scab skin samples. Different protocols have been developed for the detection of CE virus. Moreover most of these approaches are laborious, lack of sensitivity and specificity, as well as time consuming (Lard et al., 1991; Wittek et al., 1980). PCR test, has been proven by many studies as a rapid, sensitive and disease agent specific in investigating many important veterinary diseases (Abdullah et al., 2015a; Inoshima et al., 2000; Kho et al., 2000). Several PCR protocols have been described for parapoxvirus DNA detection (Mazur et al., 2000; Inoshima et al., 2000; de la Concha-Bermejiloet al., 2003; Guo et al., 2003; Tryland et al., 2005) highlighting on the avoidance of cross reactivity of serology as well as traditional culture and microscopy techniques hence the PCR is considered striking confirmatory test for CE (Abdullah et al., 2015a). The advancement of molecular biology, PCR technique has been broadly used to amplify the desired gene and had been proven as one of the most effective molecular diagnoses (Sadiq et al., 2017; Chan et al., 2007). An amplified gene products can be used as important genetic materials for further genetic analysis and gene expression studies (Razis et al., 2006; Tam et al., 2012; Ismail et al., 2012) especially for their gene functions. Furthermore, PCR could be utilized to produce complete genome sequence by closing the gaps in genome scaffold thus providing a valuable information regarding any organism (Balakrishnan et al., 2015)
PCR technique is well known to produce quick confirmatory test for ORFV. This method is recommended for proper detection and identification of pathogens by using specific gene, hence this successful confirmation of positive cases by PCR is in agreement with (Kottaridi et al., 2006; Mazur et al., 2000; Inoshima et al., 2000) whom both in separate studies confirmed CE virus detection by using major genes essential for orf virus replication.Therefore in order to ascertain prevalence and virus disease incidence rate, there is a need to combine at least two different assays for serological detection and molecular identification so as to unravel the disease status in the study population (Bala et al., 2012).
In conclusion, this study showed the presence of IgG antibody against CE virus both in sheep and goats in selected small ruminant farms in Selangor, Malaysia. It is also reported the seroprevalence rate to be higher in goats as compared to sheep. The seroprevalence rate was associated with several risk factors of which gender, age and farm factor were found to have significant effect in sheep, while clinical signs were found to have no significant effects in goats.
CONFLICT OF INTEREST
There is no conflict of interest regarding the publication of this manuscript.
ACKNOWLEDGEMENTS
The work was supported by the grant Ministry of Science, Technology and Innovation Sciencefund (MOSTI),Biotechnology Cluster 02-01-04-SF2459 (Vot no: 5450820) and GP-IPS grant of the Universiti Putra Malaysia (Vot no:9488100).
AUTHORS CONTRIBUTIONS
The authors were hereby given a declaration that this work was done by all of them named in this paper and all liabilities pertaining to claims relating to the content of this article will be borne by them. Jamilu Abubakar Bala, Mohd Lila Mohd-Azmi, Faez Firdaus Abdullah Jesse, Lim Cheng Yi, Krishnan Nair Balakrishnan, and Ashwaq Ahmed Abdullah conceived the idea, participates in data collection and run the test. Mohd Lila Mohd-Azmi, Faez Firdaus Abdullah Jesse, Mustapha M. Noordin, Abd Wahid Haron, Yusuf Abba, Isah Abubakar Aliyu, Asinamai Athliamai Bitrus, Innocent Damudu Peter, Idris Umar Hambaliand all participated in conceptualization of the idea, study design, review, and editing of paper. All authors have read and agreed with submission of final paper to the journal.
REFERENCES