Advances in Animal and Veterinary Sciences
Research Article
Effect of Cryptococcus neoformans Isolated from Pigeon on Some Hematological, Biochemical and Histopathological Parameters by Experimental Infection in BALB/c Mice
Isa Swadi Touhali
Department of Microbiology, College of veterinary Medicine, Wasit University, Iraq.
Abstract | Back ground: Cryptococcosis is the most common systemic fungal infection in humans and animals which are easily isolated from avian excreta. Therefore, it’s important to isolate them locally and to know their effect on public health by injecting them empirically into laboratory mice. Methodology: One hundred and fifty samples of pigeon droppings are collect from Wasit province, during January 2018 to March 2018.Twenty-four male adult mice type BALB/c is use, divided into two groups of 12 animals per group. One group which is consider as control, whereas other group is injected with suspension of Cryptococcus neoformans isolated from pigeon droppings as treatment group. Four animals from each group were killed every 3 days after injection serially (T1=3 days, T2=6 days, and T3=9 days respectively) to investigate the effects of C.neoformans for three injection periods on some hematological (RBC, WBC, PCV, Hb concentration, and differential WBC count), biochemical (serum ALT and AST activities) and histopathological parameters. Results: The result show twenty six droppings pigeon samples (17.3%) out of 150 samples were positive for the incidence of C.neoformans isolates and significant reduction of RBC, PCV ratio, and Hb concentration in all treated groups. While, WBC and differential WBC counts (nutrophils, monocyte and acidophil ratio) was significantly increased, while the lymphocyte and basophil ratio was reduced during the three periods of injection as compared to control. Also, the yeast suspension produced a significant elevation in the activities of serum ALT and AST with control group .but, the level of serum AST persist elevated with long injection period for 9 days than elevation of ALT comparison. As well as, data showed pathological alterations in organs (brain and lung) of mice. Conclusion: of present investigation indicates that prolong exposure of C.neoformans could mostly induced physiological alterations in blood picture, serum biochemistry and caused histopathological of brain and lung in Cryptococcus infected animals.
Keywords | Cryptococcus neoformans, Pigeon droppings, BALB/c mice
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 12, 2018; Accepted | April 02, 2018; Published | May 17, 2018
*Correspondence | Isa Swadi Touhali, Department of Microbiology, College of veterinary Medicine, Wasit University, Iraq; Email: issa.suadee@gmail.com
Citation | Touhali IS (2018). =Effect of cryptococcus neoformansisolated from pigeon on some hematological, biochemical and histopathological parameters by experimental infection in balb/c mice. Adv. Anim. Vet. Sci. 6(5): 201-206.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.5.201.206
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Touhali. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Cryptococcosis is a systemic mycosis affecting humans and a large variety of animals, subacut or chronic, life threatening caused by Cryptococcus neoformans is encapsulated yeast, an organism found in soil, often associated with pigeon droppings (Kwon-Chung et al., 2014). Cryptococcosis is considered an opportunistic fungal disease is greatly increased in immunocopromasied patients, mainly among patients with AIDS and organ transplant recipient (Heiman et al., 2011). In spite of this, Cryptococcosis is not limited to immunocompromaised persons, as shown by the current occurrence in vancover among healthy individuals (Huang et al., 2004). Cryptococcosis in animal, dog and cat to causes upper respiratory infection and skin lesion (Thomson et al., 2006). In horse, are causes sinusitis and pneumonia but in cattle, goats and sheep it mainly causes mastitis and rarely pneumonia (Duncan et al., 2006). The isolates of C. neoformans in environmental surroundings, was often isolated from bird’s excreta. Due to their high body temperature, pigeons and many such birds cannot acquire infected but can harbour the yeasts cells (Anaissie, 2002). However, the excrement of these reservoir birds is a natural defense and ideal medium for the environmental continued existence of C. neoformans and they provide in the distribution of the fungal cells (Zarrin et al., 2010). Histopathological lesions observed are with varying degrees of response in each organ depending on several factors of which the numerous properties of C.neoformans which act as an intracellular as well as an extracellular pathogen, that contribute to its virulence and help to evade the immune response (Maxie, 2007). Due to that cryptococcosis as one of the zoonotic diseases, therefore the aims of present study to isolate of C. neoformans from pigeons and its effect on public health through to our knowledge of some blood, chemical and histological changes caused by C. neoformans in laboratory mice, this study is the first in Wasit province of Iraq.
MATERIAL AND METHODS
Sampling and Isolation
One hundred and fifty samples of pigeon droppings are collect from 5 a variety of regions Wasit, Iraq , during January 2018 to March 2018.The samples are collect in sterile separate plastic test tubes and immediately transported to the laboratory in ice box. Two gram of each sample is put it in a test tube with 10 ml of normal saline, Then all tubes are shaken by vortex mixer for 5min. and left for 15 min. Culturing is made from the supernatant part in Sabouraud dextrose Agar (BDH, England) plates with chlormphenicol (200 mg/L) and incubated at 37 ºC for (3-10) days. Yeast isolates are diagnose according to cultural distinguishing, morphology culturing, shown of a capsule on India ink preparation and Urease test (Washinton et al., 2006).
Laboratory Animals
In this experiment is use white male adult mice type BALB/c at 8-10 weeks in age and weight range 20-25 gm. They are have been obtained from the College of Education/ University of Thi Qar, and maintained at the animal’s house of the faculty of veterinary medicine, Wasit University of Iraq. They are kept in mice cages and allowed free access to food and fresh water ad libitum.
Yeast Inocula
The local isolate of Cryptococcus neoformans from pigeon droppings is grow on the Sabouraud Dextrose Agar (SDA) for 48 hours at 30oC followed by a aseptically sub culturing of a heavy inoculums to tubes containing 10 ml Sabouraud dextrose broth and is incubate for 24 hours at 30oC.Then it concentrated by centrifugation 3000 rpm for 15 minutes. The yeast cells are wash three times with sterilized normal saline for 5 minutes neglecting the floating fluid each time, and diluents with sterilized normal saline to form 1:100 which are shaken smoothly. Then they are count by using a hemocytometer and re-adjusted to be 1× 106cell /ml, simultaneously. Plating for the inoculums on the SDA is conduct to assure inoculums viability (Al-Tamemy, 2010).
Study Design
Twenty four animals are divided into two groups of 12 animals per group, are use in this study. Twelve animals of first group are inject with 0.2 ml /animal (I/P) normal saline solution which consider as control group. Whereas other twelve animals injected with yeast suspension of C. neoformans in dose of 1× 106cell /animal (I/P) as treatment group. Four animals from each group are kill every three days after injection serially (T1, T2, and T3 respectively) to study physiological and histopathological parameters.
Blood and Serum Samples
Blood is collect by cardiac puncture from both control and treatment mice by using a thin small gauche syringe are dispense into sterile tube with anticoagulant for hematological examination. Blood also collected into clean non-anticoagulant test tubes and allow to colt. The serum is separate from clot and centrifuged according to animal groups into clean test tubes for biochemical analysis.
Derermination of Hematological Parameters
Determination of hematological parameters (RBC, WBC, PCV, Hb, and leukocyte differential count is perform according to (Schalm, 1975).
Derermination of Serum Biochemical Parameters
The activity of serum aspartate aminotransferase (GOT/AST) and alanine aminotransferase (GPT/ALT) is also measured in both groups (Reitman and Frankel, 1975).
Histopathological Examinatino
Biopsiesof these organs (brain and lung) are also placed immediately in 10% formalin for fixation; the histopathological sections are process according to (Bancroft et al., 1990).
Statisical Analysis
Statistical analysis is carry out using (VassrStats, 2010). All data are express as mean +/- SEM. For the analysis of the experimental parameters one-way ANOVA for independent samples followed by Tukey’s HSD Post-hoc testis use. Value of p<0.05 is consider to be statistically significant.
RESULTS
Twenty six droppings pigeon samples (17.3%) out of 150 samples are positive for the occurrence of C.neoformans isolates. The yeast colonies on Sabouraud dextrose agar is
Table 1: Effect of yeast suspension of Cryptococcus neoformans on some hematological parameters in male laboratory mice
| Parameters | Control | T1 | T2 | T3 |
|
R.B.C (10 6/µL) |
8.354+0.085 |
6.313+0.002 a |
5.847+0.001 b |
5.568+0.005 c |
|
W.B.C(10 3/µL) |
4.355+0.003 |
7.972+0.005 a |
8.047+0.006 b |
8.035+0.025 bc |
| P.C.V (%) |
30.50+0.168 |
20.65+0.064 a |
20.27+0.075 ab |
18.95+0.064 c |
| Hb (%) |
12.00+0.408 |
10.25+0.479 a |
9.22+0.259 ab |
8.42+0.246 bc |
Values are mean ± SEM, n = 4
Groups with different superscript letters are significantly different versus control group (p < 0.05).
T1: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 3days.
T2: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 6days.
T3: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 9days.
Table 2: Effect of yeast suspension of Cryptococcus neoformans on differential white blood cell count in male laboratory mice
| Parameters | Control | T1 | T2 | T3 |
| Nutrophils (%) |
56.75+0.479 |
73.00+0.408 a |
68.50+0.289 b |
66.25+0.479 c |
| Lymphocyte (%) |
36.50+0.645 |
24.50+0.645 a |
22.50+0.645 ab |
19.50+0.957 bc |
| Monocyte (%) |
3.75+0.479 |
5.00+0.707a |
6.00+0.408 b |
7.00+0.707 bc |
| Basophil (%) |
1.00+0.000 |
0.00 | 0.00 | 0.00 |
|
Acidophil (%) |
1.00+0.000 |
2.00+0.408 a |
3.00+0.707 b |
3.00+0.707 bc |
Values are mean ± SEM, n = 4
Groups with different superscript letters are significantly different versus control group (p < 0.05).
T1: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 3days.
T2: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 6days.
T3: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 9days.
Table 3: Effect of yeast suspension of Cryptococcus neoformans on some biochemical parameters in male laboratory mice
| Parameters | Control | T1 | T2 | T3 |
| GOT/AST (IU) |
35.87+0.427 |
40.85+0.064a |
39.75+0.064 ab |
38.00+0.408 c |
| GPT/ALT (IU) |
15.40+0.082 |
19.05+0.320a |
21.30+0.082 b |
22.00+0.408 bc |
Values are mean ± SEM, n = 4
Groups with different superscript letters are significantly different versus control group (p < 0.05).
T1: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 3days.
T2: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 6days.
T3: Represented animals injected with yeast suspension of Cryptococcus neoformansfor 9days.
cream colored, smooth, mucoid, and developed to wrinkled whitish – creamy colonies after further incubation for 10 days, under microscopic are yeast cells show with India ink staining appeared as oval to spherical in shape cells surrounded by capsule and neither with hyphae nor pseudohyphae, all tested Twenty six isolates are positive for the urease test and showed the ability to utilize urea as indicated by changing the medium from yellow to pink.
Hematology
The data of this study showed that yeast suspension caused a significant reduction (p>0.05) of RBC, PCV ratio, and Hb concentration in all treated comparison with control. Although, the reduced Hb concentration in all three treated periods (T1, T2, and T3) is no significant with the long injection period. While, WBC count is significantly increased in mice after administration of yeast suspension in three injection periods (3, 6, and 9 days), but the increased of WBC level is no significant between last two of injection periods (T2 and T3) (Table 1). Also, the results of differential WBC count showed a significantly increased (p<0.05) of nutrophils, monocyte and acidophil ratio, while the lymphocyte and basophilratio is significantly reduced (p>0.05) during the injection periods for 3, 6, 9 days as compared to control (Table 2).
Biochemistery
Results presented in Table 3, show that yeast suspension of C. neoformans caused significant elevation (p>0.05) in the activities of both serum ALT and AST levels comparison with control group, but the elevation of ALT activity is no significant between T1 and T2. While, the elevation of serum AST level persist with long injection period for 9 days than elevation of ALT, although high AST activity between T2 and T3 was no significant.
Histopathology Brain
Histological assessment of the brain tissues are show various intensity of inflammation with a predominance of lymphocytes, and effectively no polymorph nuclear lymphocytes. Focal meningitis-(Figure 1A) associated with infiltration of lymphocytes and mononuclear cells with focal lymphatic cuffing (Figure 1B). Marked vacuolation of the grey matter as extensive cystic spaces to cavitations are frequently observed associated with yeast infiltration which in some sections showed budding process (Figure 1C). Controls are within normal limits (Figure 1D).
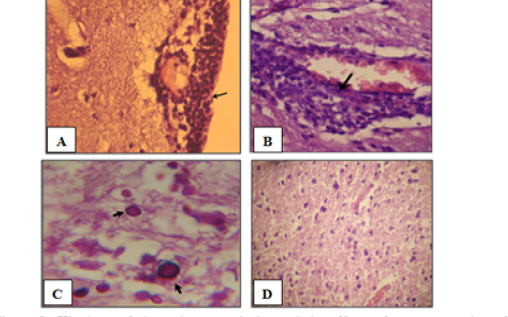
Figure 1: Histology of the male mouse brains and the effects of yeast suspension of Cryptococcus neoforman. Mice treated (T3) with dose of 1x 106 cell/animal. A) Brain, meningitis- Lymphocytes, Monocytes infiltration (H&E. staining, x400). B) Brain, lymphocytic perivascular cuffing (H&E. staining, x400). C) Brain, graymater vaculolation associated with C. neoformans infiltration (PAS ; x1000) D) Control (H&E. staining, x100)
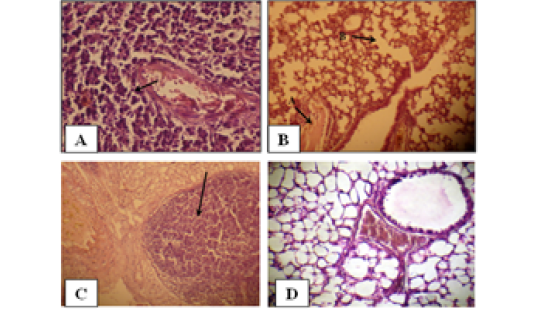
Figure 2: Histology of the male mouse lungs and the effects of yeast suspension of Cryptococcus neoforman. Mice treated (T3) with dose of 1x 10 cell/animal. A) Lung, perivascular lymphoid aggregation (H&E. staining, x400). B) Lung, (arrow A) congestion and (arrow B) emphysema (H&E. staining, x400). C) Lung, nodule of mixed inflammatory cells (H&E. staining, x100) D) Control (H&E. staining, x400)
Lung
Pulmonary sections examined. Cases of pneumonic consolidation characterize by areas of infiltration of mixed inflammatory cells, marked perivascular lymphoid aggregates (Figure 2A), dilated alveolar capillaries engorged with erythrocytes, infiltration of mixed inflammatory cells in the alveolar walls. An occasional alveoli are fill with amorphous, eosinophilic material with absence of an intraalveolar cellular filtrate also proliferation of lining epithelium in the bronchioles is observe. Congestion, edema and emphysema (Figure 2B) are abundant in most cases. There are tendency to form nodules of mixed inflammatory cells of polymorphneucleated leukocytes and mononuclear cells in the lungs of the affected animals (Figure 2). Controls (Figure 2D) are within normal limits.
DISCUSSION
The isolation of C. neoformans from the pigeon droppings in Wasit province recognized that avian habitats provide as an significant reservoir for this opportunistic pathogen and reported as essential substrates for the incidence and maintenance of C. neoformans in the environment (Majid et al.,2010). However, pigeons do not acquire cryptococcosis, most likely because C. neoformans can’t grow at the pigeon’s normal body temperature of 42 °C (Anaissie et al., 2002). The present investigation indicate that infecting mice with C. neoformansis influent, as evidenced by hematological, biochemical and histological alterations. This study showed reduction of RBC count , PCV ratio, and Hb concentration are considered due to the systemic infections, involving spread of injected C. neoformans to the blood and/or other organs in mice. This data is consistent with the report of (Muretet al.,1995).which indicated that yeast caused infection which led to a plastic anemia. Reduction in RBC count and hemoglobin concentration may be due to the direct negative action of the yeast suspension on the mice by increased rate of suppression in the rate of RBC formation and/or breakdown of red cells which support with our finding in decreased PCV. So (Shakoori et al., 1990) suggested that the decrease in RBC count is either suggestive of excessive damage to erythrocytes or reticence of erythrocyte formation. In addition, the injection with yeast suspension of C. neoformans induced increases in the number of WBC, nutrophils, monocyte and acidophil ratio, while the ratio of lymphocyte and basophilis significantly reduced. This may be refer to induction of immune response in infected mice against yeast which known that white blood cell considered first defense against different infection (Provan et al., 2009).The polysaccharide capsule of C. neoformans play a role in the immune response creation, it stimulate natural immune effectors such as natural killer cells (NK), macrophages,(nutrophils) and mononuclear cells (lymphocytes and plasma cells), While the lymphocyte in our finding is delay in increasing with prolong infection which in chronic infection. These cells consider the first line of defense in the immune system (Johnson et al., 1999). Additionally, (Rodriguez-Galan et al., 2003) refer to the role of neutrophil in controlling the spread of yeast, by limitation of yeast proliferation and invasion; Whereas notice in this investigation the acidophil is increase due to consider sign immune response for yeast infection occur increased when released histamine because destroy (Mast cells) eventually released histamine which assistant increased (WBC) especially acidophil. This study evaluated liver function for some serum biochemical characteristics by measuring the damaging effect C. neoformans on the activities of ALT\GPT and AST\GOT are manifested by increases in serum (ALT and AST) activities in comparison to control animals. This is may be due to from hepatic cellular damage by yeast suspension This finding is consistent with previous study (Nath et al., 2004) which indicated that Serum ALT and AST are also elevate with maximum elevation in yeast infected animals. ALT activity in serum results from hepatic cellular damage, the changes in the enzyme activity may also be due to vascular changes leading to hepatic vascular congestion. On the otherhand, AST is widely distributed in several tissues of the body, but the highest concentration is found in liver and muscle. So the increase in AST activity in the present study is speculatively due to liver damage. Although, the silica group showed an elevation in serum (ALT and AST).The our histopathological study showed marked pathological changes in brain and lungs organs. Brain lesions showed cavitations and this is report in other studies (Aldelem, 2003). The negligible inflammation maybe reflects the ability of this organism to efficiently avoid the host’s immune system and also the low immune resistance inside the brain (Kozel, 1996). This can explain the poor inflammatory response surrounding some of the cavities observed in the brain and the yeast cells in the tissues when stained with special staining. In order to cause meningoencephalitis, the fungal cells must endure in the bloodstream and cross the blood-brain barrier and different mechanisms are project for that (Alvarez and Casadevall, 2007). While the severity and the formation of the destructive lesions in the lung sections is suggest to be due to the phospholipas and the proteolytic enzymes produced by the C. neoformans (Da Silva et al., 2006) and that phospholipas is necessary for the entry of Cryptococcus into the pulmonary lymphatics and the blood. In addition to the capsule role current studies using murine models and macrophage-like cell lines have concerned secreted phospholipas B in intracellular survival, growth, and replication of C. neoformans within macrophages. It has been projected that PLB initiates invasion of the lung interstitial by Cryptococcus since phospholipids is the pulmonary surfactant and the outer leaflet of mammalian cell membranes are favoured substrates of the enzyme (Santangelo et al., 2004). This can explain the tissue damage in the affected organs and the profusion of the emphysematous lesions experiential in our study in many of the investigated lung sections as this enzyme can cause destruction to the alveolar walls. It has been projected that C. neoformans intracellular replication and phagocytic cells destruction are major apparatus of the pathogenic process for Cryptococcus infection in the lung and other tissues (Mitchell, 2006).
CONCLUSION
the present study show prolong exposure of C. neoformans could mostly induced physiological alterations in blood picture, serum biochemistry and caused histopathogenesis of brain , and lung in C. neoformans infected animals.
ACKNOWLEDGEMENTS
The author is grateful to all staff member of Microbiology Department College of Veterinary Medicine of Wasit University, for their help and cooperation.
CONFLICTOF INTEREST
The author declares that they have no competing interests.
Authors Contribution
All the authors contributed equally
REFERENCES





