Advances in Animal and Veterinary Sciences
Research Article
Normal Clinical Electroretinography Parameters of Healthy Pharaoh Hounddogs
Vladi Stoyanov Nedev*, Galina Petkova Simeonova
Department of Veterinary Surgery, Faculty of Veterinary Medicine, Trakia University, Student’s Campus, 6000 Stara Zagora, Bulgaria.
Abstract | Electroretinography (ERG) is a noninvasive, objective and sensitive technique with useful clinical application for evaluation of retinal function. Large number of factors, including the breed, anesthesia, environment, and equipment, has an impact on the ERG parameters in dogs; therefore, each clinic should provide reproducible ERG results for individual breed under specific conditions in order to use data for diagnostic purposes. The aim of the present study was to establish the reference values of ERG parameters in Pharao hound dog, because such data are missing in the literature. Fourteen healthy dogs (twenty-eight eyes records) were included in our study. The examination was made with a retinographic ERG unit supplied with an ERG-jet lens electrode, under general anaesthesia using medetomedine, ketamine, diazepam and isoflurane. The reference intervals (RIs) of ERG parameters of Pharao hound dog were reported, after statistical analysis of data was performed in agreement with the American College of Veterinary Pathologists (ACVP) guidelines for the determination of RIs in veterinary species.
Keywords | Elecroretinography (ERG), Pharao hound dog, Reference values
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 20, 2017; Accepted | November 23, 2017; Published | January 30, 2018
*Correspondence | Vladi Stoyanov Nedev, Department of Veterinary Surgery, Faculty of Veterinary Medicine, Trakia University, Student’s Campus, 6000 Stara Zagora, Bulgaria; Email: vladi_nedev@mail.bg
Citation | Nedev VS, Simeonova GP (2018). Normal clinical electroretinography parameters of healthy pharaoh hounddogs. Adv. Anim. Vet. Sci. 6(2): 81-87.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.2.81.87
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Nedev and Simeonova. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The full-field electroretinography (ERG) is a complex recording of electrical potentials, originated from the retinal response to light stimulation and provides information about the function of individual retinal cells (Aguirre, 1973; McLellan and Narfström, 2015). The ERG is the most important objective electrophysiological method used to evaluate retinal function in humans and animals (Ofri, 2002) and has the advantage to be a noninvasive technique. ERG is useful tool for early diagnosis of the most common retinopathies such as inherited progressive retinal atrophy (PRA), sudden acquired retinal degeneration (SARD) and optic neuritis (Narfström, 2013); as well as for monitoring of therapeutic responses and retinal toxicity of new drugs (Ropstad et al., 2007). It is also tremendously useful when performed to evaluate retinal integrity before cataract surgery (Ekesten, 2013; Wilkie and Colitz, 2013).
The determination of normal ERG values is of great importance for clinical practice, considering that there are numerous intrinsic and extrinsic factors influencing the ERG recordings including the age and breeds of the animal (Itoh et al., 2010), the environment and body temperature (Mizota and Adachi-Usami, 2002), pupil diameter, intraocular pressure and eye movement (Marmor et al., 2009; Grozdanic et al., 2010; Nair et al., 2011), electrode type and position (Mentzer et al., 2005), stage of retinal dark adaptation (Maehara et al., 2015), anesthesia technique (Norman et al., 2008; Jeong et al., 2009;Lin et al., 2009). All of that contribute to interfere with the values obtained from ERG recording; therefore, the investigations aiming to establish a normal range still continue. It is recommended each laboratory or clinic to establish a technical procedure that provide reproducible ERG results under specific conditions in order to use the data for diagnostic purposes (Ekesten et al., 2013; Sussadee et al., 2015).
Normal ERG parameters in beagles (Maehara et al., 2005), miniature schnauzers (Jeong et al., 2011), Shi-Tzu (Lee et al.,2009) poodle, Labrador retriever, Thai ridgeback, and Thai Bangkaew (Sussadee et al., 2015) were reported. The objective of our study was to establish normal ERG parameters for pharaoh dog. It is national hound of Malta existed for over 2,000 years (Parker et al., 2004) and getting more popularity nowadays. ERG investigations for this breed of dog have never been published.
Materials and methods
Animals
The inclusion criteria for the animals in this study were healthy pure-breed pharaoh dogs. Both eyes of fourteen (10 females and 4 male) ophthalmoscopically normal dogs were examined between November 2016 and March 2017. These dogs were privately owned by different owners in the central part of Bulgaria. The mean age of all dogs was 13.2 ± 8.8 months (mean ± standard error [SE]). All animals were identifiable by microchip and the pedigrees were obtained from the Bulgarian Republican Federation of Cynology.
Clinical Examination
Each dog was submitted to thorough physical examination. A complete blood cell count and standard biochemistry profile were also obtained to ensure healthy status. All dogs underwent a standard ophthalmic examination including evaluation of the pupillary light reflexes (PLRs), menace responses, blink reflexes as well as subjective testing of the ability to follow falling cotton balls in bright light and dim light conditions. Anterior segment was scrutinized using the slit lamp (Shin Nippon, Rexxam Co., Ltd., Japan). The pupils were then dilated and fundus examination was completed by indirect ophthalmoscopy (HEINE Video Omega® 2C, Germany). Afterward the ERG was made, and fundus photographs were obtained in some of the dogs at the end of the procedure.
Anesthesia
Food and water were withheld for 12 h before the anesthesia. Bright sunlight was avoided for a minimum of 2 h prior to the ERG procedure. Topical phenylephrine hydrochloride (Mydfrin ®, S.A.Alcon, Belgium) was used for maximal dilatation of the pupils by applying two drop in each eye four times with a 5-min interval between the drops. Pupil size was periodically evaluated to ensure full dilation, especially at the beginning and the end of ERG recording. Animals were premedicated with subcutaneous injection of 0.02 mg/kg atropine sulfate (Atropin Sopharma®, Sopharma, Bulgaria). The dogs were sedated with intramuscularly administered 0.02 mg/kg metedomidine hydrochloride (Domitor®, Orion Corporation, Finland) ten minutes later. Induction of anesthesia was performed with 10 mg/kg ketamine hydrochloride (Anaket®, Richter Pharma, Austria) and 0.5 mg/kg diazepam (Diazepam Sopharma®, Sopharma, Bulgaria) administered intravenously. The dogs were endotracheally intubated and inhalation was maintained with 1.0 % isoflurane (Forane®, Abbott Laboratories Limited, United Kingdom). Anesthesia was induced under ambient light. The depth of anesthesia was kept constant during the procedure. Heart, respiratory rate, and temperature were closely monitored during the procedure. Proper oxygenation and ventilation of the anesthetized dogs were maintained throughout ERG recording. At the end of the procedure, the sedation was reversed with 0.01mg/kg atipamezole (Antisedan ®, Orion Corporation, Finland).
ERG Procedure
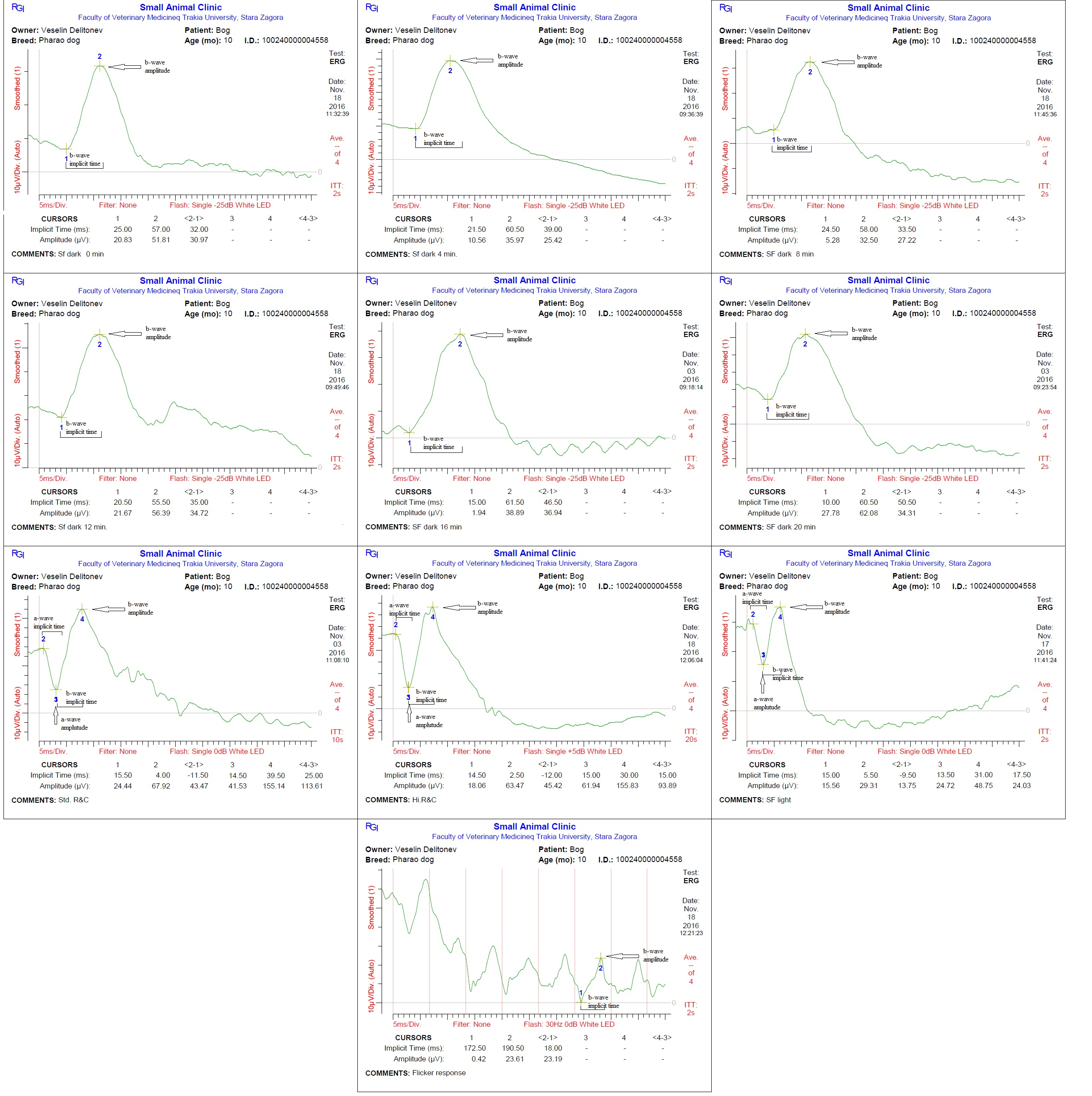
Each dog was placed in a sternal recumbent position on a mattress-covered table with the head on soft pad. Both eyelids were opened widely and an active corneal electrode (ERG-jet, Nicolet Biomedicals, USA) was positioned and 2 drops of physiological saline (Natrii chloridi 0.9%, Braun, Germany) were applied between the corneal surface and contact lens to improve conductivity. Platinum skin needle electrodes (Natus Neurology Incorporated, USA) were used as the reference and ground electrodes. The reference electrode was placed approximately 1 cm to the lateral canthi while the ground electrode was positioned over the external occipital protuberance (Figure 1). A portable mini-Ganzfeld white light-emitting diode (LED) array light stimulator (Retino Graphics, Inc., USA) was used for scotopic and photopic ERGs. It was positioned close to the eye without touching the dog. Before ERG recording, the impedance of the three electrodes was measured to ensure proper placement. Standart protocol, recommended by the European College of Veterinary Ophthalmologists (ECVO) (Ekesten Bet al., 2013) was used. First part of the examination passed in dark room for the scotopic ERG procedure (dark adaptation), which included three different responses: low intensity response using 0.01 cd.s/m2 of light stimuli every 4 minutes during 20 min of dark adaptation to evaluate rod function (SF dark); standard intensity rod and cone response (Std R&C) using 3 cd.s/m2 of light stimulus; and higher intensity rod and cone response (Hi R&C) using 10 cd.s/m2 of light stimulus. The photopic ERG procedure measured two different responses: single flash response (SF light) using 3 cd.s/m2 of light after 10 min of light adaptation with 30 cd.s/m2 of background light to assess the cone function, and 30 Hz flicker response (Flicker response) using 3 cd.s/m2 for cone evaluation while in the light adapted state. ERG data of both eyes were recorded consecutively for each dog. The recordings were analyzed with the software incorporated in the device model BPM200 ERG/VER (Retino Graphics, Inc., USA).
Data analysis
ERG waveforms were analyzed by measuring a- and b-wave amplitudes in μV (microvolt) and implicit times in
ms (millisecond) in accordance with the updated guidelines of ECVO (Ekesten et al., 2013).
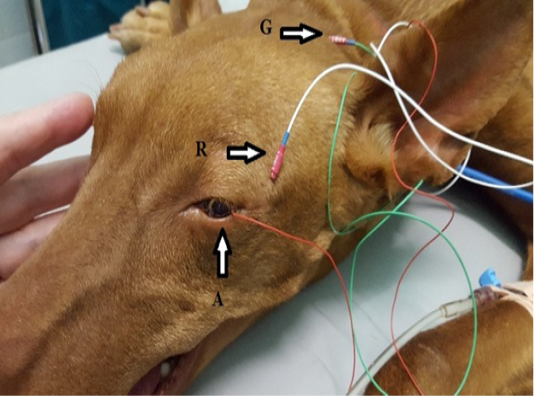
Figure 1: Position of the electrodes for ERG examination. A-active electrode in lens shape put on the cornea, R-reference needle electrode put on 1 cm of lateral eye border, G-ground needle electrode put on the external occipital protuberance.
Reference ranges of data from the respective tests were analyzed with Reference Value Advisor add-in for Microsoft Excel (Geffré et al., 2011). A Shapiro–Wilk test was performed to confirm or reject normal distribution for every measured parameter. In agreement with the American College of Veterinary Pathologists (ASVCP) guidelines for the determination of RIs in veterinary species (Friedrichs et al., 2012), the statistical method recommended for sample sizes of 20–40 with non-Gaussian distribution (e.g. robust, with 90 percent confidence intervals for upper and lower limits) was used. When outliers were identified, reference ranges were computed again after their removal from the data sets.
Results
Examination of the blood test and the eyes did not show abnormal results. ERG of healthy pharaoh dogs displayed typical for normal recording waveforms (Figure 2) with lower amplitudes comparing with other breeds of dogs. In scotopic low intensity responses, the amplitude of a-wave was undetectable, while the b-wave amplitudes gradually increased after light stimulation during 20 min of dark adaptation. The amplitudes of a- and b-waves of the scotopic standard and high intensity responses were similar. In the photopic single flash response the a- and b-waves were detectable and smaller than scotopic responses. The median, minimum, maximum, lower limit of reference interval, upper limit of reference interval, 90% CI for lower limit and 90% CI for upper limit of the implicit times and amplitudes of a-and b-waves during each individual test are summarized in Tables 1 and 2.
Discussion
It has been reported that the individual variations of ERG amplitudes is large, and therefore, it is difficult to evaluate retinal function by ERG recorded with only one recording condition (Ekesten B, 2013; Ekesten et al., 2013).
In the present study we obtained normal ERG values for Pharaoh hound dogs but with low wave amplitudes. If factors associated with dog age, examination environment, anesthesia protocol and equipment were standardized so that breed-related values could be the only concern (Ekestenet al., 2013; Mentzer et al., 2005).
The differences in ERG recordings for each breed is not fully understood but Ekesten et al. (2013) ponder that resistance and voltage of the ERG signal vary due to wide variations in skull conformation. The skull of pharaoh hounds resembles a blunt wedge, and is long and chiseled with only a slight stop and a snout of good length (Coile, 2005). The variations of skull conformation between poodles, Labrador retriever s, Thai Bangkaews, and Thai ridgebacks might be the cause for breed-specific differences in ERG parameters (Sussadee et al., 2015).
Given that the distribution and density of rod and cone cells are different between species (Peichl, 2005), it could be suggested that the same is valid for different breeds of dogs and might explain breed-specific differences.
In pharaoh dogs we found notably low values of a- and b-wave amplitudes comparatively to other breeds however all dogs had normal fundus appearance and vision. Such comparison is questionable due to differences in age, anesthetic and ERG protocols, equipment used between studies.
One major factor influencing the ERG responses is anesthesia. The standart ERG protocol established by ECVO (Narfström, 2002) was based on the International Society of Clinical Electrophysyology of Vision (ISCEV) protocol used for humans (Marmor et al., 2009), but consistent with the need for general anesthesia in animals. A study performed under different conscious conditions demonstrated that awake animals had higher a- and b- wave amplitude and shorter implicit times than anesthetized dogs. Both general anesthesia and sedation resulted in significant attenuation and delay of EGR recordings in dogs (Freeman et al., 2013).
Isoflurane has been found to decrease the amplitude of the a- and b-waves and slightly shorten implicit times for higher flash intensity stimulations (Nair et al., 2011), but other investigators reported that volatile anesthetics generally had no noticeable effect on the ERG results (Ekesten, 2007).
Table 1: Implicit time of a-and b-waves inms. Median, minimum, maximum, lower limit of reference interval, upper limit of reference interval, 90% CI for lower limit and 90% CI for upper limit are given.
| Test | n | Median (Min-max) | Reference interval | Lower 90% CI | Upper 90% CI | |
|
F dark |
24 | |||||
| b- wave | 57.15 (49-65) | 47.55-66.41 | 45.45-50.34 | 63.98-68-73 | ||
|
SF dark |
24 | |||||
| b- wave | 60.58 (50.5-104.5) | 50.68-96.50 | 48.91-53.24 | 77.93-235.45 | ||
|
SF dark |
25 | |||||
| b- wave | 60.68 (53.5-72.5) | 52.89-76.32 | 51.75-54.57 | 69.68-85.11 | ||
|
SF dark |
23 | |||||
| b- wave | 59.36 (51.5-67.5) | 51.87-68.87 | 50.11-54.01 | 65.01-70.89 | ||
|
SF dark |
25 | |||||
| b- wave | 57.73 (52-70) | 45.85-69.61 | 49.20-51.78 | 64.97-72.73 | ||
|
SF dark |
24 | |||||
| b-wave | 58.29 (51.5-68.5) | 48.92-73.56 | 47.62-51.78 | 68.09-78.10 | ||
| Std R & C | 26 | a- wave | 14.64 (12-16.5) | 12.74-21.35 | 12.29-13.56 | 16.7-32.27 |
| b- wave | 35.12 (30-47.5) | 30.18-46 | 29-31.69 | 40.73-52.67 | ||
| Hi R & C | 27 | a- wave | 15.5 (13.5-17) | 13.44-17.66 | 12.75-14.12 | 17.26-18.40 |
| b- wave | 35.61 (30-72.5) | 12.54-58.68 | 4.25-22.49 | 49.06-67.59 | ||
| SF light | 27 | a- wave | 15.05 (12-19) | 12.10-18.98 | 11.46-12.74 | 17.54-20.47 |
| b- wave | 32.31 (21-43) | 21.44-42.34 | 18.98-25.14 | 39.36-45.68 | ||
| Flicker response | 23 | |||||
| b wave | 11.29 (6.5-18) | 5.43-21.04 | 4.08-6.58 |
16.72-23.77 |
Table 2: Amplitude of a- and b-waves in μV. Median, minimum, maximum, lower limit of reference interval, upper limit of reference interval, 90% CI for lower limit and 90% CI for upper limit are given.
| Test | n | Median (Min-max) | Reference interval | Lower 90% CI | Upper 90% CI | |
|
SF dark |
21 | |||||
| b- wave | 25.69 (4.44-51.81) | -3.33-59.79 | -11.9-5.99 | 49.99-69.16 | ||
|
SF dark |
24 |
|||||
| b- wave | 25.94 (9.72-101.81) | 9.64-106.18 | 7.91-14.47 | 64.03-189.09 | ||
|
SF dark |
25 | |||||
| b- wave | 28.13 (12.22-105) | 10.56-93.23 | 9.19-13.21 | 66.87-127.21 | ||
|
SF dark |
21 | |||||
| b- wave | 32.52 (10.69-65.83) | -2.76-67.79 | -9.16-6.94 | 56.25-78.94 | ||
|
SF dark |
21 | |||||
| b- wave | 34.58 (5.97-61.53) | 2.73-66.44 | -6.48-16.58 | 57.24-83.10 | ||
|
SF dark |
22 | |||||
| b-wave | 34.97 (10.97-56.94) | 9.10-60.83 | 2.40-17.00 | 52.63-68.05 | ||
| Std R & C | 26 | a- wave | 43.36 (2.64-121.53) | -27.69-114.41 | -42.47-10.79 | 105.94-167.67 |
| b- wave | 113 (42.92-199.58) | 20.52-209.88 | -0.6-44.11 | 184.01-236.14 | ||
| Hi R & C | 27 | a- wave | 47.15 (5.14-124.58) | 5.07-119.57 | 1.17-13.36 | 97.87-149.12 |
| b- wave | 98.5 (54.44-217.08) | 55.52-232.49 | 52.92-61.49 | 178.12-277.36 | ||
| SF light | 27 | a- wave | 13.29 (5.28-24.72) | 2.35-26.39 | 0.39-4.89 | 33.8-30.23 |
| b- wave | 26.5 (9.17-70.28) | 7.93-71.04 | 6.49-10.76 | 53.06-91.67 | ||
| Flicker response | 23 | |||||
| b wave | 23.78 (5.28-50.28) | -3.85-51.42 | -10.91-3.09 | 44.01-65.72 |
Jeong et al. (2009) showed that a combination of xylazine and ketamine has the lowest impact on implicit times and amplitudes without ventral rotation of the eyeball and miosis. But this combination should not be used in elderly or high-risk patients because of severe cardiovascular suppression caused by the first agent. That is why we replaced the alpha-2 agonist xylazine with a safer one medetomidine. Mild to moderate sedation with medetomidine reduces flash ERG a- and b-values (Norman et al., 2008).
With regard to diazepam, it alters retinal function solely by affecting amacrine-ganglion cell interactions and reduces the a-wave amplitude (Stafanous et al., 1999).
In our anesthetic protocol we used the before-mentioned agents in low doses to provide a safe general anesthesia suitable for elderly or ill animals with presumably negligible effect on the values of ERG components. The same anesthetic and ERG protocol was used to define the normal ERG parameters in another breed of dog (Nedev and Simeonova, 2017). The differences between pharaoh and Bulgarian hound dogs were obvious but still low amplitudes could be seen in the litter breed. Therefore, the explanation should be addressed to some technical concerns such as the type and position of electrodes.
Mentzer et al. (2005) discovered that the distance of a reference electrode from the examined eye and the type of active electrode determined the magnitude of wave amplitudes.In all previously cited studies reporting higher than ours amplitudes, the distance between active and reference electrode was greater. But in order to achieve the best noise reduction, the distance between electrodes should be as small as possible (Narfstrom et al., 2002).
In conclusion, normal ERG parameters of Pharaoh hound dog were established using a safe anesthesia protocol and handheld multi-species ERG unit. More data need to be accumulated to clarify the reason for breed differences in ERG components.
Acknowledgments
This study received no support from any funding organizations.
Conflict of interest
We the authors, declare that there is no conflict of interest.
Authors contribution
Vladi Nedev performed ERG recordings while Galina Simeonova maintained animal`s vital parameters under general anaesthesia. Both authors took part in writing and discussing the subjects of the articles.
References