Advances in Animal and Veterinary Sciences
Research Article
Serological Survey of Aujeszky’s Disease in Peninsular Malaysia in 2016
Suet Ee Low1, Peck Toung Ooi1*, Nor Yasmin Abdul Rahaman1, Sheau Wei Tan2, Zi Herk Cheah3, Eric Heng Chow Cheah3, Evonne Pei Qin Lim3, Chiun Khang Yong3, Kok Yen Kam3
1Faculty of Veterinary Medicine, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia;2Institute of Bioscience, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia;2Animal Health Division, BoehringerIngelheim Malaysia Sdn Bhd, 50490, Kuala Lumpur, Malaysia.
Abstract | Aujeszky’s disease (AD) is a common swine disease that widespread throughout the world. The symptoms include nervous signs, respiration and reproduction problems that lead to great economic losses to the industry. AD is endemic in Malaysia, where outbreaks had been reported in previous years. In Malaysia, approximately 95% of the pig farms are vaccinated for AD. Despite the regular vaccination, AD serological status remains unknown in this country. This study provides AD serological status in Peninsular Malaysia in 2016 based on the samples submitted to Faculty of Veterinary Medicine, University Putra Malaysia (UPM). A total of 1154 serum samples from 36 farms were submitted for AD ELISA diagnostic test; grouped as 8 weeks, 12 weeks, 16 weeks, 20 weeks, gilts and sows with different parity. The samples were subjected to AD antibody detection with IDEXX Pseudorabies Virus gpI Antibody Test Kit. Among the 36 farms submitted to UPM, 8 farms were detected with positive gI antibody indicated that these farms were still facing challenges from AD field virus. Among these eight seropositive farms, three farms were having seroprevalence in the range of 33.33% to 37.14%. In general, vaccination of AD is ideal and stable in Malaysia but we still need to be alert with the field challenge as it will be a threat to the industry.
Keywords | Aujeszky’s disease, Serological status, Pig farm, Malaysia, ELISA
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 07, 2017; Accepted | January 24, 2018; Published | January 30, 2018
*Correspondence | Peck Toung Ooi, Faculty of Veterinary Medicine, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; Email: ooi@upm.edu.my
Citation | Low SE, Ooi PT, Rahaman NYA, Tan SW, Cheah ZH, Cheah EHC, Lim EPQ, Yong CK, Kam KY (2018). Serological survey of aujeszky’s disease in peninsular malaysia in 2016. Adv. Anim. Vet. Sci. 6(2): 75-80.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.2.75.80
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Low et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Aujeszky’s disease (AD), which also known as pseudorabies or ‘mad itch’, is a viral disease resulting in no table economic impact to the swine industry (Freuling et al., 2017). It is caused by Suidherpesvirus 1. Often, the causative agent of AD is also known as Aujeszky’s disease virus (ADV) or pseudorabies virus (PRV). The virus is categorized under family Herpesviridae, subfamily Alphaherpesvirinae in genus Varicellovirus (Andrewes, 1963; Mettenleiter, 2000). It is an enveloped double stranded DNA virus containing genome approximately 150kbp which comprised of unique long region (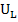 ) and unique short region (
) and unique short region (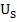 ). Infection of AD involved withawide range of hosts, in exception of equine, primates and humans. All susceptible mammals are dead-end hosts and variably fatal once infected with the disease, except for pigs. Pigs are the only reservoir host that able to survive even productive infected with the disease (Mettenleiter, 2000).
). Infection of AD involved withawide range of hosts, in exception of equine, primates and humans. All susceptible mammals are dead-end hosts and variably fatal once infected with the disease, except for pigs. Pigs are the only reservoir host that able to survive even productive infected with the disease (Mettenleiter, 2000).
Transmissions of AD normally take place via airborne, oral route including contaminated water and colostrum from the infected sows to suckling piglets, and mosteffective way is the direct contact between pigs. It can also be transmitted via vaginal mucosa or semen upon breeding between infected pigs (Beran, 1991). Infection of ADV on pigs can occur at different production phase. Morbidity rate, mortality rate and clinical signs of AD infection upon its severity vary in age and immunity of pigs (Nauwynck, 1997). The mortality rate is high among infected newborn piglets withseveral nervous system signs shown.Infected older pigs and pregnant sows are mostly suffered from respiratory signs and reproductive failure respectively. Latency characteristic of herpes viruses has contributed to the ability of AD to persist in the infected pigs for a lifetime. This has always been the biggest concern for the eradication of AD. Reactivation and dissemination of the virus could be induced subsequently in carrier pigs, by means of survived or recovered from the infection. Stress and hormonal imbalance will trigger the onset of disease in carrier pig (Wittman et al., 1983; Zimmerman et al., 2012). Tonsils, olfactory bulb, trigeminal and sacral ganglia are the four main sites of virus latency (Romero et al., 2003; Wheeler and Osorio, 1991).
In Asia, China was the first country reported with AD outbreak in the1950s (Li and Guo, 1994), then it was spread to other countries such as Taiwan in 1971 (Lin et al., 1972), Japan in 1981 (Fukusho, 1982) and South Korea in 1987 (Kim et al., 1988). Neighbour countries were also subsequently reported with outbreaks including Singapore (Koh et al., 1979) and Thailand (Sunyasootcharee et al., 1978) in 1977 and Philippines in 1985 (Marero, 1985). An outbreak of AD in Malaysia occurred in 1976 (Lee et al., 1979) and the number of AD cases involved in anabruptincrement in 1977. However, AD still not declared as an endemic diseasein our country at that time. It’s strongly believed that the existence of AD in Malaysia was originated from the import of infected pigs. AD was declared as endemic in Malaysia after an AD outbreak happened in 1984 (Too, 1997). Approximately 95% of Malaysia pig farms are practising vaccination of AD nowadays.
The vaccine has been developed as apreventive measure against field virus infection (Van Oirschot, 1999), whereby it induces the build-up of immunity in the animals by protecting them from the challenge of field virus infections. Hence, efficacy and effectiveness of vaccines are essential components to look at when it is being used to control any animal diseases (Meeusen et al., 2007). When marker vaccine was developed in the 1980s, itis able to discriminate the immunity response developed from infection or vaccine. Marker vaccine of AD is available in inactivated and attenuated live forms and both types of vaccines are available in Malaysia. A study has proven that vaccinated pigs are less susceptible to field infection, as high amount of AD virus is required to initiate an infection. In addition,vaccinated herd also excreted less virus load compare to unvaccinated populations (De Smet et al., 1992; Pensaert et al., 2004). Therefore, vaccination has played an important role in protection of a population. It also contributes in elimination of the disease gradually.
In order to control AD in a more effective way, a rapid detection of viral infection via confirmation of the laboratory diagnosis is important as observations based on clinical signs might be insufficient in identifying AD accurately. Several diagnostic tests are applicable for detection such as particle concentration fluorescence immunoassay (PCFIA), latex agglutination (LAT) test, virus neutralization test (VNT) and enzyme-linked immunosorbent assays (ELISAs). ELISA is an ideal test because it requires lesser time and provides excellent sensitivity and specificity.
The latest serological status of AD reported in Malaysia was done nearly two decades ago (Jasbir, 1998). Limited data are available about the field virus infection and serology of AD in Malaysia. Therefore, this study is to update the current situation of AD disease status in the pig farms and provide information about the AD serological status in Malaysia in 2016.
RESULTS
A total number of 1,531 commercial serum samples comprising of 49 farms were received for AD serological diagnosis in Faculty of Veterinary Medicine, Universiti Putra Malaysia, throughout the year of 2016. However, a number of 13 farms with 377 samples (26.53%) are sampled without meeting the age group category, whereby 1,154 of serum samples from 36 farms were only eligible with the age group criteria. These 36 farms consisted of 58.33% (21/36) of northern region farms, 11.11% (4/36) of central region farms and 30.56% (11/36) of southern region farms.
After all these 1,154 samples were screened with ELISA in detecting the presence of gpI-antibodies, it was found out that 95% of the samples were reported with seronegative results. Only four samples were reported under the category of uncertain (0.347%). Meanwhile, 4.25% (49/1154) of samples were detected with positive for antibodies to glycoprotein I (gpI) indicating the presence of field virus strain of AD challenge in the farm.
As an overview on the farms, a number of 28 farms (77.78%) were reported with 100% seronegative result of gpI antibodies out of the 36 farms which indicated free from AD field challenge. Inversely, there were 8 farms (22.22%) detected with suspected or positive gpI antigen in their farms. These eight farms were included two farms (N06 & N07) from the northern region and three farms each from central (C02, C03, & C04) and southern (S02, S06, & S11) regions. Central region farms had most of the farms infected (75%) with AD field virus strain while northern region had the least farm of infection (9.52%).
Base on the findings, the northern region farms had the lowest gpI antigen detection rate (Table 1). Both seropositive pig farms in northern region had seroprevalencerate of 2.86% and 4.0%. Meanwhile, the seroprevalence of three seropositive gpI antigen farms in central region were ranged from 8.11% to 20%. For southern region farms, three sampled farms showed the highest field challenge detection rate. In all these three affected farms, the field challenge rates were all higher than 30%. On the other hand, in the comparison between the age groups it regardless of the region, seropositivity of each age group was similar to each other. The age group with the lowest seropositivity was 8 weeks old pigs (1.31%) whereas sows have the highest seropositivity (6.40%).The result indicated that the condition might be age-related, as older animals have higher seropositive rate compare to younger animals (Table 2).
Table 1: Percentage of seropositive samples of each gE/gpI detected farms in each region.
| Region | Farm Code | Percentage of seropositive samples |
| Northern | N06 | 4.00% |
| N07 | 2.86% | |
| Central | C02 | 8.57% |
| C03 | 8.11% | |
| C04 | 20.0% | |
| Southern | S02 | 33.33% |
| S06 | 37.14% | |
| S11 | 34.29% |
DISCUSSION
To date, there is not much of serological study reported about AD from commercial pig farms in Malaysia. The last seroepidemiological study of AD in Malaysia was conducted back in 1998 (Jasbir, 1998). There is no updated study regarding serological status of AD for over nearly two decades. Therefore, this study is crucial in contributing information to control and prevent the disease.
Gene E deleted AD vaccine has been a very good marker vaccine for overcoming the difficulties to differentiate field infection and vaccination (Pensaert et al., 2004). Differentiation of the sources of induced antibody response from the animalscaused by vaccine or infection is very important because it can provide crucial information in order to conduct an effective serological surveillance (Freuling et al. , 2017). Marker vaccine of AD was developed by deleting glycoprotein E (gE) for its live and killed vaccine. The other nomenclature for gE is known as gpI where the latter was an old nomenclature that still can be seen in several literatureof previous years (World Organization for Animal Health (OIE), 2012). Pigs vaccinated with gE/gpI deleted vaccine would develop immunity response without gE/gpI while pigs exposed to field AD virus strain would be detected with gE/gpI in their antibody responses. Therefore,thepresence of gE/gpI antibody responses has served as an indication for the presence of field virus strain in the farms. The detection of presence or absence of gE/gpI antibodies by using ELISA was developed by Van Oirschot et al. (1986) where the method has been applied to most of commercial ELISA test kit for AD. IDEXX PRV/ADV gI, a commercial ELISA test kit, is used as the diagnostic tool in this study for the detection of gpI antibodies in the serum samples. It is a reliable test which is as good as SNT and can be used as an alternative way in diagnosing for serological purposes due to its high sensitivity and specificity up to 96.25% and 98.75% respectively (Jasbir, 1998). Detection of antibodies to gE/gpI in the samples can also due to vaccination of AD vaccine that containing gE/gpI strain. However, AD vaccination in Malaysia has been carried out using gE/gpI deleted marker vaccine in the commercial pig farms. Thus, every positive result obtained will indicate the challenge from field virus strain.
In general, the country is still not completely free from the field challenges because a small number of sampleswere detected with seropositive of field type AD infection in our study (4.25%).Currently, AD field strains challenges are low in Malaysia, as low detection rate and no serious AD cases reported. Back in the year of 1998 until now, the number of AD field virus infection had reduced 51.15% for the pig population sampled and 61.78% for the pig farms surveyed in Malaysia (Jasbir, 1998). This data could be further confirmed by the improved situation of pig farms in Malaysia. Occasional AD outbreaks had been often reported in different areas of the country back in 1990’s, whereby this disease had brought up great concern among the farmers for the issue during that time. It also created awareness among farmers towards this disease as it had led to huge impact and economic losses on the pig production in those days. However, sporadic outbreak for AD was less to be seen or reported in Malaysia pig farms in recent years. Hence, we could say that field virus challenges of AD have gradually become lesser in Malaysia.
Northern region has the least infection rate compared to other regions. Seropositivity of field AD virus strain challenge in the infected farms of northern region was also the lowest among all other positively detected farms in any other regions, signifying the presence of field AD virus challenge but not a high-level challenge in the region. Penang and Perak are two of the major pig producing states in Peninsular Malaysia categorized under northern region. However, the infected farms were only reported in Penang, none in Perak. In other words, an inference can be drawn is that Perak state is currently free from field virus
Table 2: Percentage of seropositive samples of northern, central and southern region farms in each age group
| Region | Percentage of seropositive samples (%) | |||||
| 8 weeks | 12weeks | 16 weeks | 20 weeks | Gilt | Sows | |
| Northern | 1.11 (1/90) | 0 (0/90) | 0 (0/89) | 0 (0/90) | 0 (0/82) | 0.51 (1/195) |
| Central | 0 (0/16) | 0.06 (1/16) | 0 (0/16) | 0 (0/16) | 0 (0/17) | 0.24 (9/37) |
| Southern | 0.02 (1/46) | 4.08 (2/49) | 6.12 (3/49) | 10.42 (5/48) | 19.51 (8/41) | 11.46 (11/96) |
| Total | 1.32 (2/152) | 1.94 (3/155) | 1.95 (3/154) | 3.25 (5/154) | 5.71 (8/140) |
6.40 (21/328) |
infection of AD. Based on our samples, both seropositive farms in Penang were actually managed by the same owner and did not exhibit any clinical signs of AD. Thus, it was highly suspected that the seropositive detection in Penang pig farmswas possibly obtained via latency of the virus. Based on the serological outcome of northern region, it shows that field virus spread was probably very low in both of the states or could even possibly absent in Perak state. This might be contributed by good biosecurity practice and ideal vaccination regime applied in the region’s pig farms.
The percentage of detection of AD field virus infected farm was the highest in central region (75%) compared to other regions. However, the percentage of seropositive of AD field virus strain in the infected farms of central region was not the highest but lies in between the range of northern and southern region. This result indicates that AD field virus challenge was widespread in pig farms of the region but the infection within the farms were under control. Since most of the farms in central region were having detection of AD field virus challenge, it could be deduced that this might be regional circumstances. A high number of pig farms detected with antibody responses against field virus strain antigen in the region was most probably due to the high density of pig farms in central region, where numerous farms were concentrated in specific areas. Study has proven that highly dense pig farms or close proximity between pig farms in a region are accounted for the increase of seropositive percentage of herds (Boelaert et al., 1999; Hu et al., 2015). In details, the risk of circulation and transmission among the pig farms increased when a minimum of 10,000 pigs population was found living in the distance of 6km (Tamba et al., 2002). Another study had reported that a distance of 2.5km could be in a risk (Rodríguez-Buenfil et al., 2002).The factors behind proximity between farms and herd size associated positively with seropositivity are possibly airborne transmission of virus and indirect contact of movement of transportation or human in the farm. This is because airborne transmission of ADV could take place up to 10km in distance (Casal et al., 1997; Leontides et al., 1994a). Moreover, positive association were reported between seropositivity risk and location of pig farms that near to water sources, such as lake or river, because high level of humidity could induce aerial transmission of ADV to be much effective (Solymosi et al., 2004). Most of the pig farms in central region were located nearby the sea and the area will be misty during early morning when the weather turns cold. Therefore, the environment around pig farms in central region could be one of the contributing factors towards the high AD field virus infection risk of the farms.
For southern region, the result obtained was in converse with the result for central region. The risk of infection of farms was considered low (27.27%) in southern region. However, seropositivity within the infected farms was the highest among all other infected farms, of which the percentage had reach up above 30% in the region. This result shows that the extent of AD transmission between farms was not high in the region but circulation of AD field virus infection within the farm itself was notably high. Unlike central region, pig farm densities in southern region are low as pig farms are dispersed individually in the states. Thus, it can deduce that the existing issue in southern region is not much related to its spatial but more likely towards farm management issue. One of the gE/gpI positive farms was located in Malacca while the remaining two were reported from Johor. Both seropositive Johor pig farms are under the management of the same owner but they are located in a different area in Johor. Based on the scenario, it is speculated that biosecurity, virus spread in the area and vaccination are the three factors in affecting the risk of infection in these farms. Transportation of pigs or human between farms could contribute to the transmission of field virus from one farm to another (Austin et al., 1993; Martínez-López et al., 2009), of which attributed to poor biosecurity practices in the farms. Similar high seropositivity in both infected Johor pig farms was probably due to this factor. Introduction of wild-type AD strain into the farm would be due to the purchase of field ADV infected gilt (Leontides et al., 1994b). It is believed that it could be one of the factors in contributing towards the detection of positive gI/gpI antibody response 80% of gilts sampled in the farms.
CONCLUSION
In general, AD condition is considered under control and not much of a threat in Malaysia, as low AD field virus detected and no serious AD problem reported in the country. Different patterns of serological result were reported from northern, central and southern region. Northern region had the least AD infected farms and lowest number of seropositive pigs in the infected farms. Central region had the most AD infected farms while southern region had the highest seropositivity within the infected farms. These results indicate that current AD vaccination program is stable and effective in providing protection to pig farms in Malaysia. Therefore, it is important for farmers to maintain AD vaccination and keep monitoring on the farm status. We still need to be alert with the field challenge as it will be a threat to the industry.
MATERIALS AND METHODS
Samples Collection
Convenient samples were sent to University Putra Malaysia for routine monitoring where samples were obtained from commercial farms. Throughout 2016, a total of 1531serum samples were received comprised of 49 farms for PRV gI serological diagnosis. The collected samples were comprised of 8 weeks, 12 weeks, 16 weeks, 20 weeks, gilts and sows. Generally, 3 to 5 samples were collected for each age group and 10 samples were collected for sows of various parities. Information such as the pig population, the structure of the farm, health history of pigs and vaccination schedule for AD were taken from each farm.
Region Categorization
The pig farms in five main states of Peninsular Malaysia were categorized according to their geographical location into three regions which are the northern, central and southern region. Penang and Perak belong to northern region, Selangor is under central region, while Malacca and Johor are categorized into southern region in this study.
AD Antibody Detection
Enzyme-Linked Immunosorbent Assay (ELISA) test was applied in this study to detect antibodies towards gpI antigen of Aujeszky’s disease virus (ADV) by using Pseudorabies Virus gpI Antibody Test Kit (IDEXX Laboratories, Inc., Westbrook. US). By following manufacturer’s protocol, the ELISA test was initiated with dilution of test samples including positive and negative control with sample diluents for 1:2ratios.Then, all diluted test samples, positive and negative control were dispensed into the ELISA plate for 100 µl and incubated for one hour. After the incubation, the ELISA plate was washed with wash solution for 3 to 5 times and dispensed with 100µl conjugate to each well. Incubation was taken place for 20 minutes and the similar ELISA plate washing was repeated. After that, 100µl of TMB substrate was dispensed and incubated for 15 minutes. Lastly, 50µl of stopsolution was dispensed into each well and read with the ELx808™ Absorbance Microplate Reader. The results were interpreted with IDEXX x ChekPlus® software.
Result Interpretation
The S/N value was obtained by converting optical density of samples according to the manufacturer’s calculations.
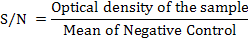
Positive, suspected and negative results were determined according to the range established by manufacturer’s specification. Negative result was reported when the S/N value obtained is greater than 0.70 while positive result was reported when the S/N value obtained is lesser or equal to 0.60. Suspected result was reported when the S/N value obtained was in the range greater than 0.60 and lesser or equal to 0.70.The positive result of antibodies detection in serum samples indicates the presence of field strain AD virus challenges in the farm or exposure of vaccine with gpI antigen.
ACKNOWLEDGEMENTS
The authors would like to express gratitude to BoehringerIngelheim (Malaysia) Sdn. Bhd and Geran Putra -Inisiatif Putra Siswazah (IPS) (GP-IPS/2016/9507500) for technical and financial support in conducting this study.
CONFLICT OF INTEREST
The authors declared that conflict of interest is absent in the conduct of this study.
AUTHORS CONTRIBUTION
LSE conducted the research work and data interpretation. OPT, NYAR and TSW contributed to methodology design and manuscript editing. CZH, ECHC, ELPQ, YCK, KKY contributed in samples collection.
REFERENCES





