Advances in Animal and Veterinary Sciences
Short Communication
Variation Factors of the Pregnancy Rate of Arab Pure Breed Mares Inseminated by the Deep Intracornual Method in Post-ovulation
Najjar Amel1, Khaldi Sana1,2, Ben Said Samia3, Hamrouni Abir1, Benaoun Belgacem3, Ezzaouia Mohamed3
1Laboratory of Animal, Genetic and Food Resources, National Institute of Agronomy, Tunisia; 2National School of Vetarinary Medicine of Sidi Thabet, Tunisia; 3High School of Agricultural of Kef, Tunisia; 4National Foundation for the Improvement of The Horses Breeds, Sidi Thabet, Tunisia.
Abstract | This work was aimed to study the influence of different factors on the pregnancy rate in the Pure Arab Breed (PAB) mares, inseminated intra-cornually in post-ovulation. A total of 62 thoroughbred PAB mares were inseminated by this method during the breeding season of 2016. Almost half of these mares (28) received an hCG- treatment (Chorulon®) to induce ovulation. Heat in all the mares was detected by rail teasing, and their follicular activity monitored 3 times a day by ultrasound (9am, 4pm and 11pm) until observation of the corpus luteum. At this moment, intracornual artificial insemination with frozen semen (AIF) is executed and the pregnancy rate is determined after the pregnancy confirmation examination, at 35 days post AI. Results showed that the pregnancy rate does not vary between the group of mares treated with Chorulon® (37%) and that of untreated mares (34%). However, this parameter varies significantly according to the moment op AI (21.5% if the AI occurs at 4pm versus 36% and 36.5% respectively if the AI is practiced immediately after the 9am and 11pm ultrasound examinations, respectively, p<0.05). Moreover, the rate of pregnant mares is higher when the ovulation occurs in the right ovary (p<0.05). A significantly higher number of pregnancies is obtained when the diameter of the preovulatory follicle measures between 35 to 44 mm (p<0.05). The identified variation factors influencing the pregnancy rate in PAB mares are the moment of insemination, the ovary where the ovulation occurs, and the diameter of the preovulatory follicle.
Keywords | Pregnancy rate, Deep insemination, Post-ovulation, Factors, Mares
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 21, 2017; Accepted | January 06, 2018; Published | January 10, 2018
*Correspondence | Najjar Amel, Laboratory of Animal, Genetic and Food Resources, National Institute of Agronomy, Tunisia; Email: amelnajarbenmatoug@gmail.com
Citation | Najjar A, Khaldi S, Hamrouni A, Ben Said S, Benaoun B, Ezzaouia M. (2018). Variation factors of the pregnancy rate of arab pure breed mares inseminated by the deep intracornual method in post-ovulation. Adv. Anim. Vet. Sci. 6(1): 40-43.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.1.40.43
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 A-Najjar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Deep intracornual artificial insemination (AI) improved the pregnancy rate of mares in stud farms. However, this method requires a rigorous monitoring of the ovarian activity of the mare and a high technicality of the practitioner. Despite the gains of this method of insemination, variations affect the pregnancy rate of the mares during breeding seasons. Certain factors implicated in this variation are identified, such as the age of the mare (Davies Morel and O’Sullivan, 2001), the number of spermatozoa per insemination dose (Sieme et al., 2004), the type of semen (chilled or frozen) used for insemination (Crowe et al., 2008), the frequency of monitoring ovarian activity (Lemma et al., 2006, Newcombe et al., 2011) and the diameter of the preovulatory follicle (Arbel et al., 2015).The objective of this study is to study the effect of some factors influencing the rate of pregnancy in Pure Arab Breed (PAB) mares inseminated by the deep intracornual method, with frozen semen during the Tunisian breeding season.
MATERIAL AND METHODS
Generalities
This study took place in the National Foundation for the Improvement of The Horses Breeds stud farms of Sidi Thabet, located in northern Tunisia, 24 km from the capital Tunis (36° 54′ 31″ north, 10° 02′ 33″ east) . A sample of 62 purebred Arabian mares was followed during the 2016 breeding season, from February 15th to May 30th. Mares are housed separately in individual boxes and received the same diet (33% greenery, 27% barley, 20% straw and 20% hay).
Ovaries Ultrasound
Heat detection in mares occurs daily at 7am using teasing rails and a teaser stallion (Haras Nationaux, 2004). Ultrasound monitoring of the follicular activity starts on the third day of estrus, and is performed daily on both ovaries with an ultrasound instrument (Aloka 500®) provided with a 5 MHz linear probe. Ultrasound examination involves locating the preovulatory follicle in generally one of the two ovaries, and determining its diameter each time (Miro, 2012). As soon as the follicular diameter reaches 35 mm, the ultrasound examination becomes thrice a day (7am, 4pm and 11pm).
Mares are divided into two groups according to the measurement of the preovulatory follicle diameter: those with a preovulatory follicle diameter between 35 and 44mm, and those with a wider preovulatory follicle (diameter> 44mm).
Hormonal Treatment
A group of mares (n = 28), known for the irregularity of their cycles, received hormonal injections to solve this problem. Indeed, an intramusculary injection of 5ml of Chorulon®(INTERVET) whose active ingredient is a synthetic hCG, and whose activity is LH-like, at a concentration of 1500 IU, causes an ovulation to occur in 24 to 36 hours (Samper, 2009).
Artificial Insemination (AI)
Artificial insemination with frozen semen (AIF) is carried out immediately after the ultrasound examination revealing a corpus luteum. The AIF is intraconrual with the deposit of the semen at the junction the uterine horn and the entrance of the oviduct ispsilateral to the corpus luteum: this method is called deep AI in post-ovulation (Andrew, 2011). The recommended dose per AI in this case is 100 million spermatozoa.
Pregnancy Diagnosis
The pregnancy diagnosis is performed at an early stage of the gestation (14 days post AIF) by ultrasound. A second ultrasound examination is carried out at 35 days post AIF to confirm the pregnancy, and the rate of pregnancy in mares is calculated by dividing the number of pregnant females by the total of the inseminated ones.
Statistical Analysis
Data variance analysis is performed by the SAS software (SAS Institute Inc. ®), using the General Linear Model procedure (GLM). Chi 2 test is used to compare the percentage of pregnant mares that did or not undergo a hormonal treatment, the percentage of pregnancies issued from ovulation in the left or right ovary, and the groups of mares with different diameters for the preovulatory follicles (35 to 44 mm and >44mm). Statistical significance threshold is set for p<0.05.
RESULTS AND DISCUSSION
The number of mares inseminated in April and May is more important than in March and even more so in February. However, pregnancies occurred more often in April than in March and May (Figure 1). The reduced number of AI registered in February is explained by the beginning of the breeding season, and by the number of the mares resuming their ovarian activity (Najjar, 2011). The same explanation is relevant regarding the number of pregnant mares in February. Indeed, pregnancy confirmation is executed starting on March for mares inseminated in February.

Figure 1: Variation of the number of inseminated mares and pregnant mares according to the month of the breeding season.
The rate of pregnant mares varied from 21.5% when the AI is done after the ultrasound examination at 4pm to 36 and 36.5% respectively when AI is practiced after the 9am and 11pm ultrasound examination (Table 1). Newcombe et al. (2011) showed that the timing of the post-ovulation AI performed on mares that underwent only 2 ultrasound examinations spaced by 12 to 15 hours does not improve the rate of pregnant mares. In our study, the pace of ultrasound examinations is more intense, and the rate of pregnant mares calculated after each examination is acceptable, especially since our results showed that the inseminations performed in the morning (9am) and evening (11pm) have improved the rate of pregnant mares. This is probably related to the time of ovulation.
Table 1: Variation of the rate of pregnant mares according to the moment of AIF.
| Moment of the AIF | 9 am | 4pm | 11 pm |
| Rate of pregnant mares (%) | 36.5 | 21.5 | 36 |
AIF : Artifical Insemination using Frozen semen
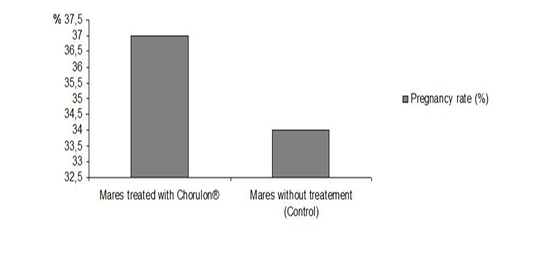
However, it should be noted that the rate of pregnant mares did not vary between the group of mares hormonally treated with Chorulon® (37%) and the control sample (34%) (Figure 2). This finding is not consistent with the results of Samper (1997). In fact, in her experience, the injection of hCG was performed during the heat phase, and subsequently followed by an ultrasound examination, triggering an ovulation 48 hours later. The injection of hCG was followed by the decrease of the endometrial edema (Samper, 1997). Chorulon® allows the induction of ovulation and does not improve the rate of pregnant mares, by impacting the folliculogenesis.
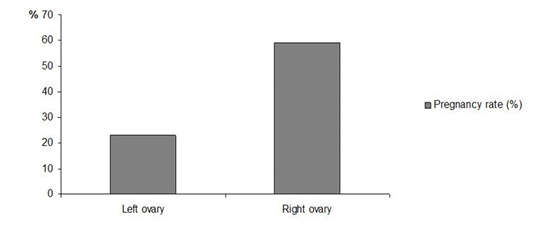
Regarding the influence of the ovulation site (right or left ovary), our results show that there is significantly more pregnancies if the ovulation occurs in the right ovary (59% pregnancies are issued from an ovulation in the right ovary versus 23% from the left ovary, p<0.05, Figure 3). Davies Morel and O’Sullivan (2001) and Rizagholizadeh et al. (2015) reported that the two ovaries of the mare function alternately, and that there is no difference between their ovulation rates. Nevertheless, Fukuda et al. (2000) demonstrated that the ovulation rate of the left ovary favors a better pregnancy rate in a study on the ovarian activity in women.
In addition, preovulatory follicles with a diameter between 35 and 44 mm gave a higher rate of pregnant mares compared to the ones with a larger diameter (> 44 mm) : 87% versus 23% respectively (p <0.05, Figure 4). These results agree with those reported by Ginther et al. (2008) and Arbel et al. (2015). Indeed, the latter found that the rate of pregnant mares is significantly higher with a preovulatory follicle of 40 mm in average.

Figure 4: Variation of the pregnancy rate in mares depending on the diameter of the preovulatory follicle.
CONCLUSION
This experiment allowed the improvement of the pregnancy rate in mares when inseminated by the deep intracornual method with frozen semen in post-ovulation, in the morning and in the evening, suggesting that ovulation probably occurs during these periods. Moreover, ovulations occurring in the right ovary, and a preovulatory follicle diameter between 35 and 44 mm gave the best pregnancy rates. This study enables us to conclude that the rate of pregnant mares varies according to the time of insemination, the site of ovulation and the diameter of the preovulatory follicle.
ACKNOWLEDGEMENTS
The authors of the manuscript are thankful to the National Foundation for the Improvement of The Horses Breeds and the Laboratory of Animal, Genetic and Food Resources of the National Institute of Agronomy of Tunisia, for their support during this study.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS CONTRIBUTION
Najjar Amel, Khaldi Sanaand, Ben Said Samiaparticipated in the experiment and wrote the manuscript. Ben Said Samia and Hamrouni Abir carried out the statistical analysis. Ezzaouia Mohamedand Benaoun Belgacem supervised the field trial.
REFERENCES