Advances in Animal and Veterinary Sciences
Short Communication
Management of Fetal Mummification/Maceration Through Left Flank Caesarean Section in Cows - Study of Four Cases
Ravi Dutt1*, Jasmer Dalal1,Gyan Singh2, Subhash Chand Gahalot1
1Department of Veterinary Gynaecology and Obstetrics, LUVAS, Hisar, Haryana-125004, India; 2Department of Veterinary Clinical Complex, LUVAS, Hisar, Haryana-125004, India.
Abstract | The present study was conducted on 4 Gynaecological cases in cows presented to Department of Veterinary Clinical Complex, (VCC), LUVAS, Hisar with history of over gestation and suffering from dystocia due to fetal maceration (two cows) and foetal mummification (two cows).All animals were already treated at field level for induction of parturition with PGF2α and Dexamethasone 72-96 hours before reporting to VCC but no response was observed. On per vaginal examination, two animals were found with closed cervix and two had 2 fingers open cervix. All animals were examined through trans-rectal ultrasonography and confirmed pregnant with scanty fluid in contracted uterus and fetal skeleton. Initially, all animals were treated for induction of parturition with Valethamate bromide, Estradiol benzoate, PGF2α, and Calcium boro-gluconate. But no response was observed after 24 to 48 hrs of treatment in cervical dilatation. So, it was decided to perform laparohysterotomy through flank approach in all cases as uterus was contracted around small fetus which was difficult to exteriorise with ventro-lateral incision parallel to milk vein in lateral recumbency. In all animal fetuses were exteriorised successfully. Post-operative treatment with fluids and antibiotics was recommended for 7 days. All animal except one with severe uterine adhesion recovered and became cyclic.
Keywords | Caesarean section, Cow, Dystocia, Left flank, Maceration, Mummification
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 17, 2017; Accepted | December 11, 2017; Published | December 28, 2017
*Correspondence | Ravi Dutt, Department of Veterinary Gynaecology and Obstetrics, LUVAS, Hisar, Haryana-125004, India; Email: raviduttvets@yahoo.co.in
Citation | Dutt R, Dalal J, Singh G, Gahalot SC (2018). Management of fetal mummification/maceration through left flank caesarean section in cows - study of four cases. Adv. Anim. Vet. Sci. 6(1): 12-16.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.1.12.16
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Dutt et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A farm economy depends upon yielding a calf per year per cow. Among various gestational disorders, fetal mummification and maceration form important cause of failure to achieve the target and imposes huge economic loss by extending the inter-calving period as well as fetal loss (Azizunnesa et al., 2010). Fetal mummification and maceration have been described most frequently in large ruminants and mostly occur after the 1sttrimester of gestation (Roberts, 1971). Fetal mummification is characterized by fetal death without concomitant luteolysis and adequate cervical dilation (Kumar et al., 2013). In cattle, the incidence of fetal mummification is reported to be about 0.13-1.8% (Barth, 1986) and occurs most frequently between three and eight month of gestation (Kumar et al., 2013). Most of the mummified fetuses remain in the uterus until treatment is given to expel out or remove by caesarean section (Wenk off and Manns, 1977). The choice of treatment of fetal mummification is injection of prostaglandin PGF2α and in non responsive cases caesarean section is opted.
Fetal maceration is common sequelae of mummification and generally occurs in the event of death of fetus after formation of the fetal bones, regression of corpus luteum and failure of abortion (Arthur et al., 1989). Incomplete abortion after the third month of gestation is the main reason for a retained fetal bony mass in the uterus of cows and buffaloes (Sood et al., 2009).
Unlike mare (Burns and Card, 2000) and small ruminants (Mehta et al., 2005; Ajitkumar et al., 2007), the condition is more common in cattle and buffaloes (Purohit and Gaur,
Table 1: Physiological body parameters in animals suffering from fetal maceration and mummification.
| Parameters | Animal 1 | Animal 2 | Animal 3 | Animal 4 |
| Breed | Sahiwal | Crossbred | Hariana | Jersey |
| Parity |
2nd |
3rd |
2nd |
2nd |
| Age (years) | 5.5 | 7 | 6 | 5 |
| Body condition score | 4 | 2.5 | 3 | 3 |
| Body Temp. (°F) before treatment | 100 | 102 | 105 | 104 |
| Heart rate before treatment | 68/min | 65/min | 86/min | 72/min |
| Respiration rate before treatment | 18/min | 16/min | 36/min | 21 |
| Body Temperature (°F) after treatment | 101 | 101.5 | 103.5 | 102 |
| Heart rate after treatment | 72/min | 70/min | 94/min | 76/min |
| Respiration rate after treatment | 16/min | 18/min | 26/min | 16/min |
| Colour of mucous membrane | Pinkish | Slightly pale | Highly congested |
Congested |
Table 2: Gynaecological observations, treatment and outcomes
| Parameters | Animal 1 | Animal 2 | Animal 3 | Animal 4 |
| Pregnancy period (Days) | 313 | 332 | 360 | 295 |
| Cervical dilatation (fingers) |
External os (1) Internal os (closed) |
External os (2) Internal os (closed) |
External os (2) Internal os (2) |
External os (3) Internal os (2) |
| Uterine Adhesion | Not present | Not present | Severe | Negligible |
| Vaginal discharge (amount, colour and smell) | Slight, mucoid and normal | Slight, turbid and normal | Moderate, Reddish-brown and foul smell | Intermittent, brownish-yellow and foul smell |
| Previous treatment at field level | PG + Dexamethasone | PG + Dexamethasone | PG + Dexamethasone | PG + Dexamethasone |
| Response of above treatment | Nil | Nil | Nil | Nil |
| Fetal characteristics | Mummified | Mummified | Macerated | Macerated |
| Treatment Given at University clinic |
C/S through left flank
|
C/S through left flank | C/S through left flank | C/S through left flank |
| Recovery and resumption of cyclicity | Recovered and resumed cyclicity after 60 days | Recovered and resumed cyclicity after 60 days | Died after 20 days of surgery |
Recovered and resumed cyclicity after 30 days |
2011). The available literature describes the management of macerated fetus by manual removal of fetal bones per vaginum through a dilated cervix (Purohit and Gaur, 2011; Mehta et al., 2005). However, laparohysterotomy for removal of the macerated fetus is potentially dangerous and must be considered only as a last resort (Honparkhe et al., 2008).The objective of the present study was to assess the survivability and future fertility of four cows after delivery of mummified/macerated fetuses through laparohysterotomy (caudal left flank) which was remained non responded previously to medicinal treatment.
Materials and Methods
Present study was conducted on four cows of different breeds those were presented in the Department of Veterinary Clinical Complex of LUVAS with a clinical history of prolonged gestation having mummified (Animal no 1 and 2) and macerated fetuses (Animal No. 3 and 4) in utero. All the cows were inseminated artificially and confirmed as pregnant trans-rectally by field Veterinarian but failed to deliver the fetuses at the expected time of parturition.
Examination and Data Recoding
Physiological body parameters (temperature, heart rate, respiration rate), the data related to breeds, age, parity and gestation period were recorded just before study (Table 1). Physiological body parameters of all the animals were found to be normal, except in Haryana cattle (Table 1). Appetite, posture and gait were normal in all the cows.
Reproductive Examination
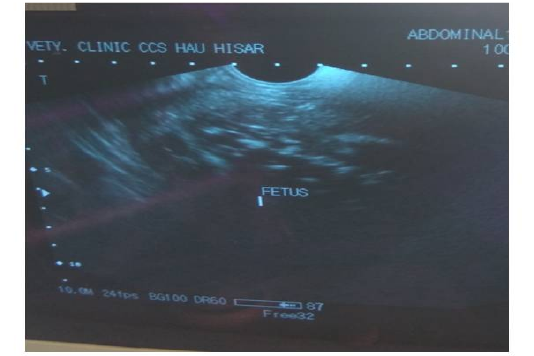
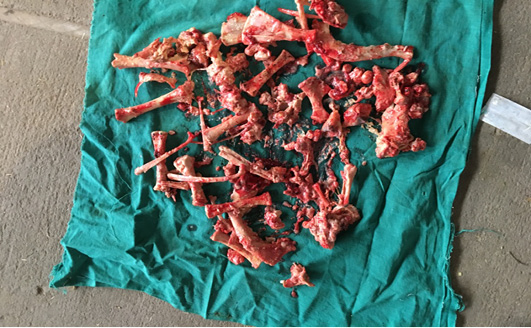
Trans-rectal palpation revealed absence of fetal reflexes and tightly contracted uterus around the fetus in both animal no 1 and 2. There was a hard mass inside the uterus but the placentomes, fremitus and fetal fluid were absent on per-rectal palpation. Per vaginal examination indicated closed cervix in both the cases (Table 2). The cases were subsequently revealed as fetal mummification in trans-rectal ultrasonography (Figure 1). Trans-rectal ultrasonography was performed using real–time B-mode (5.0-7.0MHz). Ultrasonographic examination showed the fetus as a compact, immobile mass without placental fluid or placentomes and absence of a heartbeat (Lefebvre , 2014) indicative of mummification. However, in animal no 3 and 4, fetal bones with remaining tissues floating in pus in uterus and a crepitating sound was experienced on per-rectal palpation. On per vaginal examination, cervix was found open and pus discharge was observed (Table 2). The case was subsequently diagnosed as fetal maceration.
Procedure
Initially, all animals were treated for induction of parturition with Valethamate bromide (Inj. Epidosin, TTK health care, 80mg, IM), Oestradiol benzoate (Inj. Preg Heat, Virbac, 2mg, IM), Prostaglandin (Inj. Vetmate, Vetcare, 500µg, IM) and calcium boro-gluconate (Inj. Mifex, Novartis, 450 ml IV). But no response was observed in cervical dilatation after 24 to 48 hrs of treatment in all animals. So it was decided to perform caesarean section through flank approach.
Preparation of Animal
Broad spectrum antibiotics (Inj. Amoxirum forte, Virbac India Ltd, 4.5g IM), fluid (Dextrose saline 5%, 3 liter IV) and vitamin (Inj. Belamyl, Zydus cadila Ltd, 10ml IM) therapy was administered to each animal prior to surgery for stabilization. The area of left paralumbar fossa e.g.ventral and caudal to lumber process was selected for operation in all animals and the area was prepared for operation by clipping, shaving and sterilizing with chlorhexidine and spirit solution. Paravertebral regional anaesthesia as described by Weaver (1986) and Kumar (1996) and light sedation was given as mentioned by Kumar (1996). Lignocaine hydrochloride 2%was used for paravertebral regional anaesthesia and Xylazine (0.5) ml was given intramuscularly for light sedation only in Hariana cattle. During operation 3liters of 5% Dextrose normal saline was infused intravenously to compensate dehydration from fluid and blood loss.
Operative Procedure
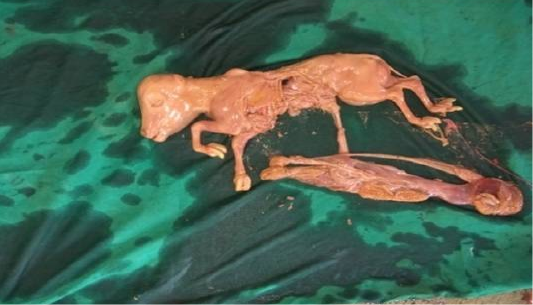
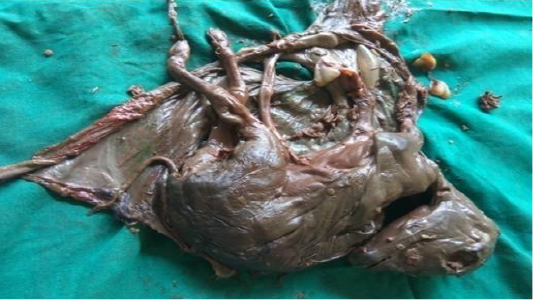
Following aseptic preparation of the operative field, a 14 inches long vertical incision along the caudal border of flank was made to open the abdomen as described by Noordey (1994). A careful dissection of skin, muscles and peritoneum exposes uterus. The distended gravid uterus was pulled out through the incised opening (Figure 2). In all the animals, the uterus was exteriorised easily except in one (animal No. 3) with severe uterine adhesions with peritoneum and visceral organs. Drapes and sterile gauge were used to prevent the leakage of uterine fluid into the peritoneal cavity. A longitudinal incision (Figure 3) along the greater curvature of uterine horn was made to remove the mummified (Figure 4 and 5) and macerated fetuses (Figure 6). All the remaining parts of fetus, placenta and bones were removed carefully and uterine cavity and peritoneal cavity was flushed with diluted povidone iodine solution.
Animal No. 4 (Jersey) lied down in the cattle crush during the operation and was taken out of crush and further operative procedure was carried out successfully in sternal recumbency. Other three animals stood normally during surgery. Incised uterus was sutured with chromic catgut no.3 in two layers by Lambert and Cushing pattern while deep muscles layers along with peritoneum were sutured in lock stitch pattern with chromic cat gut no 3. Superficial muscles were sutured by simple continuous pattern taking deep layers in alternate suture bite to remove dead space. Skin wound was opposed by silk in horizontal mattress pattern.
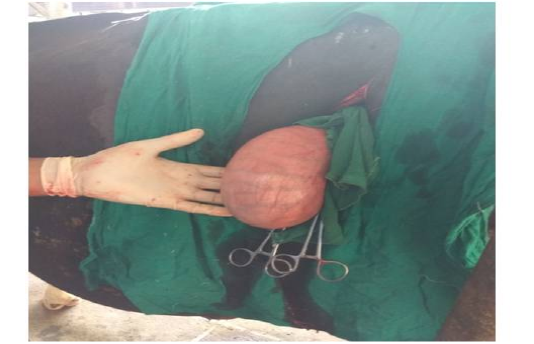
Figure 2: Exteriorised uterus of animal No. 2
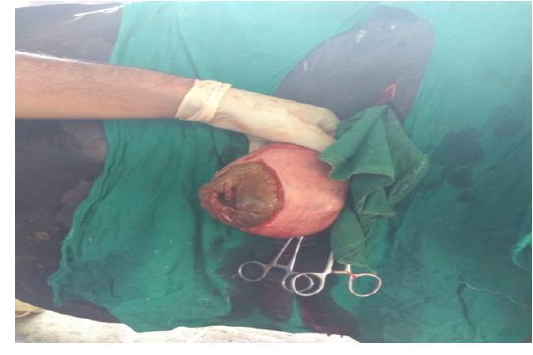
Figure 3 Incised uterus of animal No. 2
Post-operative
Just after operation Oxytocin (Inj. evatocin, Neon laboratories Ltd.10ml IV) was given in 1 litre normal saline to accelerate uterine involution. The main concern was deputed to prevent the possible toxaemia, alleviate the inflammatory pain and to check the secondary infection or complications. Dextrose saline 5% (3 litres/day) was continued intravenously for 5 days after operation to reduce the risk of toxaemia, to minimize stress, and energy expenses. Amoxicillin-sulbactum (Inj. Amoxirum forte, Virbac India Ltd, 4.5g IM) injected in all animals for 7 days to prevent bacterial infection. Flunixin meglumine (Inj. Megludyne, Virbac India Ltd, 20ml IM) was given for 7 days used as anti-inflammatory. B-complex (Inj. Belamyl, Zydus cadila Ltd, 10ml IM) was injected daily up to 5 days. Owner was advised for removal of skin sutures after 14 days of surgery and telephonic conversation was made with respective owners to assess the final outcomes.
All owners were contacted regular intervals and according to them, the appetite and general appearance of the 3 animals were satisfactory with apparent signs of recovery for the first few days. The animal was kept in clean and dry place and was supplied adequate balance feed. Three animals recovered around 15 days post operation. One animal with macerated fetus along with severe uterine adhesion died with peritonitis symptoms. As per information obtained from owners, incision site had healed completely, temperature, rumination, condition of the uterus, reproductive tract and other related physical parameters were normal in all. After 2 months of operation, all the three animals showed regular oestrous cycle but the animals were not inseminated at first post-partum heat (Table 2). The peak milk yield went up to 10-15 kg in all the recovered animals.
Discussion
Mummification is generally associated with a well-developed corpus luteum. The treatment of choice remains as induction of luteolysis by injection of PGF2α, that follows the expulsion ofthe mummified fetus within 2 to 4 days (Jackson and Cooper, 1977). Occasionally a certain percentage of animals do not respond to the treatment and caesarean section is wise option to deliver the fetus (Bhuyan et al., 2016). Present cases of mummified fetuses were also not responsive to the treatment and Caesarean section in these animals was done as describe by Bhuyan et al .(2016). As the uterus is generally tightly contracted around fetus in mummification, caudal flank laparotomy is suitable choice for caesarean section that was also used in this study (Lefebvre et al., 2009). Future fertility was good in both animals as they become cyclic within two month of surgical operation.Similar result was found by Roberts (1986). He noted that cows usually conceive on the 1st or 2nd estrous cycle after expulsion of mummified fetus. In literature management of macerated fetus include per vaginal removal of bony pieces through a dilated cervix (Kumar et al., 2013). When the cervix is not open, prostaglandins or estrogens can be given to regress the partially regressed CL and to increase the uterine contractions (Purohit and Gaur, 2011). However, in the present cases of maceration, there was cervical non dilatation even after 24 to 48 hr of treatment, hence laparohysterotomy through caudal flank, had to be performed as described previously (Purohit, 2012). Survivability of animal is severely compromised when laparotomy is used for removal of an macerated fetus as infection to peritoneum may occur during the operative procedure. This also happened in present case; one of two animals (Animal no 3) collapsed after 20 days of surgery owing to poor vital indicators before the operation while another (Animal no 4) recovered successfully and become cyclic within one month.
Conclusion
The fetal mummification and maceration are important gestational disorders in which the exact etiology and time of fetal death are not known. The duration of illness and condition of animal decides the survivability and future fertility of dam. In cases of incomplete dilatation of cervix after hormonal therapy casearean section seem to be last resort. In caesarean section through ventro-lateral incision parallel to milk vein in lateral recumbency, there are chances of seepage of contaminated uterine content in abdominal cavity and difficulty in suturing the comparatively smaller size of uterus than normal pregnancy. Therefore, delivery of macerated /mummified fetus through left flank approach is often advantageous.
Acknowledgements
Authors are highly thankful to Head, Department of Veterinary Clinical Complex, LUVAS, Hisar for providing facilities to carry out the surgery.
Conflict of Interest
There is no conflict of interest to declare.
Authors Contribution
Ravi Dutt was involved in anaesthesia management, and manuscript writing. Jasmer and Gyan Singh performed the surgery and Subhash Chand Gahalot was involved in final proof reading of the manuscript.
References