Advances in Animal and Veterinary Sciences
Research Article
Molecular and Serological Detection of Newcastle Disease Virus in Native Chickens from Selected Live Bird Markets in Batangas, Philippines
Gil John O. Resplandor, Dennis V. Umali*
Department of Veterinary Clinical Sciences, College of Veterinary Medicine, University of the Philippines, Los Baños, Laguna, 4031, Philippines.
Abstract | At present, little information is known about the distribution of Newcastle disease virus(NDV) in live bird markets (LBMs) in the Philippines. Serological and molecular detection of NDVs in native chickens from different LBM in the Philippines were performed. Results showed that out of 49 sera tested, 48 (97.96%) were positive for NDV with hemagglutination-inhibition (HI) antibody titers ranging from 22 to 29 and geometric mean titer of 26.62.Except for respiratory signs, minor physical injuries and non-specific lesions, all native chickens did not show clinical signs of ND at the time of examination. Nested RT-PCR was performed on 76 oropharyngeal and cloacal swab samples and 49 pooled tissue samples. All results were negative for NDV. The unusually high HI antibody titers against NDV and the negative nested RT-PCR results may suggests that the examined birds may have been previously exposed to virulent NDVs but may have already recovered from clinical disease. Regular monitoring through disease surveillance and physical inspection of LBMs in the country should be regularly implemented to decrease the risk of transmitting NDV to the commercial poultry industry and prevent any future ND outbreak in the Philippines.
Keywords | Hemagglutination inhibition, Live bird markets, Newcastle disease, Philippine native chickens
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 05, 2017; Accepted | November 14, 2017; Published | November 25, 2017
*Correspondence | Dennis V Umali, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, University of the Philippines, Los Baños, Laguna, 4031, Philippines; Email: dvumali@up.edu.ph
Citation | Resplandor GJO, Umali DV (2018). Molecular and serological detection of Newcastle disease virus in native chickens from selected live bird markets in Batangas, Philippines. Adv. Anim. Vet. Sci. 6(1): 1-7.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.1.1.7
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Resplandor and Umali. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Newcastle disease (ND) is a highly devastating disease of domestic poultry and wild birds (Alexander, 2000; Alexander 2009; Alexander et al., 2012). It is caused by Newcastle disease virus (NDV), also known as Avian paramyxovirus serotype-1 (APMV-1). This virus belongs to genus Avulavirus, subfamily Paramyxovirinae, family Paramyxoviridae and order Mononegavirales (Alexander, 2000; Alexander 2009; Alexander et al., 2012; ICTV, 2017; MacLachlan and Dubovi, 2011). NDV is a negative sense single stranded, non-segmented enveloped RNA virus. The NDV genome is approximately 15.2 kb long composed of 6 genes that codes for nucleocapsid protein (NP), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin-neuraminidase (HN) and RNA polymerase (L) (Alexander, 2000; Alexander 2009; Alexander et al., 2012; OIE, 2017).
Based on clinical manifestation of the infected chickens, NDV can be grouped into five pathotypes namely viscerotropic velogenic, neurotropic velogenic, mesogenic, lentogenic and asymptomatic (OIE, 2017). NDV can be also classified into Class I or Class II based on the genomic size and nucleotide sequence of F and L genes (Alexander, 2000; Diel et al., 2012; Umali et al., 2013). Class I strains are mostly avirulent to chickens while Class II NDV strains are comprised of mostly virulent strains to chickens and are further subdivided into several genotypes and subgenotypes (Alexander, 2000; Alexander 2009; Alexander et al., 2012; Diel et al., 2012; Umali et al., 2013; Miller et al., 2015).
Live bird markets (LBM) play important roles in the spread of velogenic NDV strains (Jibril et al., 2014). Based on location of LBM, it can be classified into urban, rural or village and roadside live bird markets (Solomon, 2011). Movements of village chickens (Spradbrow, 2000) and multi species set-up in LBM provide a conducive environment for ND transmission (Molia et al., 2015). The LBM in the Philippines selling birds of multiple species and ages comprise a considerable portion of the poultry industry. Birds being sold in Philippine LBMs vary in species from native to commercial chickens, game fowls, pigeons, ducks, turkeys and quails. Majority of these birds are either poorly vaccinated or unvaccinated and may act as reservoirs of NDV strains. LBMs are also a concern because these provide a favorable environment for the transmission of NDV through direct contact with contaminated cages, food, water, feces and especially that natural reservoir host of ND like waterfowls are marketed in close proximity to domestic gallinaceous species.
The Philippine native chickens are consist of a number of local breeds of chicken in the Philippines. The native chickens in the Philippines are said to be descendants of domesticated red jungle fowl (Gallus bankiva) (Bondoc, 1998). Native chicken has been an integral part of the rural setting where it is mainly raised to become an additional source of income to the farmers. An adult male can weigh for about 1.3 kilogram and the adult female is 1.0 kilogram. The meat and eggs of native chickens are preferred by many Filipinos because of its taste, leanness, pigmentation and suitability for special dishes (Bondoc, 1998). These native chickens have other unique attributes including adaptability to harsh environment, ability to utilize farm by-products and resistance to diseases and parasites (Bondoc, 1998). The province of Batangas is one of the most densely poultry populated province in the Philippines. As of 2016, Batangas produces around 102,612 tons of chicken meat and 106, 256 tons of eggs or around 6.13% and 23.01% of total national production respectively (PSA, 2017). Any eventualities of disease outbreak of economically important poultry pathogens especially in Batangas will adversely affect poultry production in the Philippines.
In spite of the importance of ND in the Philippines, no studies have been conducted yet regarding the status of NDV in Philippine native chickens. Knowledge and understanding on the distribution of NDVs in LBMs in the Philippines is relatively little. LBMs possess continuous risk of transmission of NDV to domestic poultry, hence detection and characterization of NDVs in LBM is highly essential to understand the current status of NDV in Philippine native chickens and role of LBMs in transmission of NDV. Molecular and serological surveillance of NDV may provide valuable information on the epidemiology of the disease, which may be important in the formulation of more effective ND treatment and control strategies in the Philippines.
MATERIALS AND METHODS
The use of animals in this study as described in the procedure below was approved by the Institutional Animal Care and Use Committee (IACUC) of the College of Veterinary Medicine, University of the Philippines Los Baños with assigned protocol number 2017-0011.
Live Bird Markets
Live bird markets used in this study were identified through the 2014 Live Bird Market Survey of Batangas, which was obtained from the Regional Animal Disease and Diagnostic Laboratory (RADDL) of Region IV-A. Based on the population size and type of operation, four live bird markets specifically from the municipalities of Rosario, Padre Garcia, Lemery and Tanauan were selected. Relevant information using prepared questionnaire were obtained to characterize the operations of each LBM.
Philippine Native Chickens
A total of 49 Philippine native chickens were randomly collected from the selected LBM. Sample distribution were as follows: 15 from Rosario, 14 from Padre Garcia and 20 from Lemery, Batangas. Selected birds were purchased and brought to UP-Veterinary Teaching Hospital for sample collection and necropsy. In addition, oropharyngeal and cloacal swab were also randomly collected from native chickens on site. These chickens were selected through the consent of the vendors and the availability of chickens on the time of the collection. A total of 10 birds were swabbed on Rosario, 15 birds from Lemery and 2 birds from Tanauan, Batangas.
Sample Collection
All chickens were individually weighed and checked for any clinical signs of disease. The eyes, nares, ears, integument, feet and vent were examined for any lesions. Oropharyngeal and cloacal swab were individually collected on the birds using sterile cotton swabs. Swabs were immediately placed in 2 ml normal saline solution containing penicillin at 10,000 units/mL and streptomycin at 10 mg/mL. Using 3 ml 23 gauge syringe, at least 6 ml of blood was collected via wing vein. Sera were harvested and transferred to Eppendorf® tubes. Both the swabs and sera were stored at -20°C until further analysis. All 49 chickens were sacrificed through intra cardiac injection of air. The following organs such as lungs, trachea, liver, spleen, cecal tonsils, bone marrow, thymus and bursa were collected during necropsy and stored in individual air-tight plastic containers at -20°C until testing.
Table 1: Profile of the selected LBM in Batangas, Philippines
| Location | Transport of birds | Schedule and Volume of Opreations | ||
| Municipalities (Barangay) | Origin | Destination | Day (Time) | Approximate Volume (birds/day) |
|
Lemery (Maguhan) |
Lemery San Luis Cuenca Alitagtag Agoncillo San Nicolas Taal Sta Teresita Bauan San Pascual Rosario |
Banay Banay, Lipa Dasmarinas, Cavite Manila San Luis Taal Mindoro Sta Teresita Tagaytay Aranque Lemery |
Wednesday and Saturday (4-10 AM) |
300-800 birds |
|
Tanauan City (Barangay 7) |
Mabini Tinurik Janopol Oriental |
Local Talisay Santo Tomas Malvar |
Everyday (5AM-7PM) | 25-50 birds |
| Rosario (Poblacion East) | Rosario | Rosario | Friday (5-6:30AM) | 300-500 birds |
|
Padre Garcia (Poblacion) |
Padre Garcia Rosario |
Padre Garcia | Friday (4-6AM) |
250 birds |
Serological Detection
The antibody titer of native chickens against NDV were determined through Hemagglutination Inhibition (HI) test as described by the OIE (2017). In summary, 25µl phosphate buffered saline was dispensed into each well of a plastic U-bottomed microtitre plate. Two fold serial dilutions of 25 µl chicken and negative control sera were performed. Twenty-five (25) µl of 4 HA unit (HAU) NDV LaSota antigen was added and mixed to each well. The plate was incubated for a minimum of 30 minutes at 37C. Twenty-five (25) µl of 1% washed RBCs was added and mixed to each well. The mixture was left to stand at 37C for 30 minutes. Complete inhibition of hemagglutination was determined as the endpoint titer. The antibody level for each serum sample was expressed as a log base 2. Tests were performed in duplicates and replicated three times.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
At least five grams of lungs, trachea, liver, spleen, cecal tonsils, bone marrow, thymus and bursa of Fabricius were pooled and homogenized using sterile mortar and pestle. Homogenized tissue samples were mixed at a concentration of 30% with normal saline solution containing penicillin at 10,000 units/mL and streptomycin at 10 mg/mL. Mixture was centrifuged at 6000 rpm for 10 minutes. Viral RNA from pooled swab and homogenized tissue samples were extracted using QIA amp®. Viral RNA Mini Kit (Qiagen, West Sussex, UK) according to the manufacturer’s instructions. Extracted viral RNA were transcribed to complementary DNA (cDNA) using random hexamers and reverse transcriptase (Takara Bio-Inc,Shiga, Japan). A two-step nested RT-PCR was performed to amplify the region comprising the 3′ end of the M-gene and the 5′ end of the F-gene using SapphireAmp Fast PCR Master Mix (Takara Bio-Inc,Shiga, Japan) (25µL/50µl reaction volume), external and internal primers (0.2µM) and template (~50-100ng) as described previously (Mase et al., 2002; Umali et al., 2013; Umali et al., 2014; Umali et al., 2015).The PCR product of both first and second PCR were analysed using gel electrophoresis. The gel was prepared using 1.2 % gel stained with Gel Red (Biotium Inc., California, USA).
RESULTS
Characteristics of the LBMs
The detection of NDVs in Philippine native chickens from LBMs in Batangas, Philippines using hemagglutination inhibition (HI) test and nested reverse transcriptase polymerase chain reaction (RT-PCR) were performed. To the best of our knowledge, this is the first study to detect NDVs and to characterize LBMs in the Philippines. The characteristics of four LBMs located in Rosario, Padre Garcia, Lemery and Tanauan in the province of Batangas are presented (Figure 1 and Table 1). It was observed that LBMs in Batangas had various practices. The LBM in Lemery is located in an open public space near the public market while the LBM in Rosario and Padre Garcia are both located along the major roads near the public market. In Tanauan LBM, the vendors sell their live birds inside the public market where they have designated stalls. There are only specific time and day of the week when these LBMs operate. The trading and selling of live birds happen
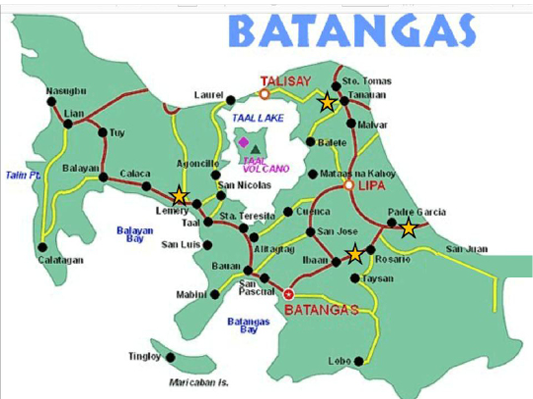
Figure 1: Map of the province of Batangas showing the four municipalities were the study was conducted. LBMs in the study were marked with a star
as early in the morning. The LBM in Lemery, Batangas operates from 4 to 10 AM during Wednesday and Saturday while both LBMs in Rosario and Padre Garcia operate from 5:00-6:30AM every Friday. Vendors in these LBMs have no fixed stalls and they sell their birds along major roads and public spaces in close proximity to public markets (Figure 2). The LBM in Tanauan operates every day from morning until evening and is located in a fixed area in the public market. Different live birds are being sold like ducks, pigeons, game fowls, turkeys, culled layers, and native chickens. They are usually placed on netted cages, chicken baskets and wired baskets (Figure 2). It was observed that different bird species are mixed together in one cage. The supplies are brought in the site by the local native chicken rearers who acts as the seller too. Most of the birds being sold in the LBMs originate from the different barangays and municipalities near the location of the LBMs. There are viajeros who buy in bulk in one LBM and sell the purchased live birds in another LBM or in their own locality in another province or city. Other buyers are individuals who buy one or two chickens for personal consumption.
Clinical Findings
All of the 49 Philippine native chickens from the LBMs of Batangas were unvaccinated. Physical examination showed that three out of the 49 native chickens had feather picking lesions on the saddle area. Ten native chickens had mucoid nasal discharge. Three native chickens had broken nares and three chickens had bloody traumatic wounds on the vent area. Necropsy findings showed that three native chickens from Padre Garcia and eight native chickens from Lemery had multifocal tumorous lesion in the intestines. Aside from respiratory exudates, none the chickens examined had clinical signs suggestive of velogenic ND.
Serological Test Results
Out of 49 birds, 97.96% of the birds (48) showed high HI antibody titer against NDV. Geometric mean antibody titer was 26.62 with a range of 22 to 29 (Table 2). Table 3 shows the summarized frequency distribution of the NDV antibody titers.
Table 2: NDV HI titers of native chickens from the different LBMs of Batangas, Philippines
| LBM | HI Antibody Titers (log2) | Number of chickens |
| Rosario | 5 | 1 |
| 6 | 4 | |
| 7 | 7 | |
| 8 | 3 | |
| Padre Garcia | 2 | 1 |
| 4 | 1 | |
| 5 | 1 | |
| 6 | 2 | |
| 7 | 4 | |
| 8 | 5 | |
| Lemery | 5 | 4 |
| 6 | 2 | |
| 7 | 5 | |
| 8 | 8 | |
| 9 | 1 | |
| Positive Rate | 97.96 % | |
| Geometric Mean Titer | 6.62 |
Molecular Test Results
Out of 76 swab samples and 49 tissue samples collected from the different LBMs, all tested negative for NDV using RT-PCR (Table 4).
DISCUSSION
The nature of LBMs plays a crucial role on possible viral transmission of ND and other diseases (Molia et al., 2015). There are several studies conducted that showed how NDV
Table 3: Frequency distribution of NDV HI antibody titers of native chickens from the different LBMs of Batangas, Philippines.
| HI Antibody Titers | Number of Native Chickens | Percent (N=49) |
|
21 |
0 | 0.00% |
|
22 |
1 | 2.04% |
|
23 |
0 | 0.00% |
|
24 |
1 | 2.04% |
|
25 |
6 | 12.24% |
|
26 |
8 | 16.33% |
|
27 |
16 | 32.65% |
|
28 |
16 | 32.65% |
|
29 |
1 | 2.04% |
|
210 |
0 | 0.00% |
| Total | 49 |
100% |
Table 4: Results of nested RT-PCR of pooled swab and tissue samples of native chickens from the different LBMs of Batangas, Philippines.
| Sample | Pooled Swabs (N=76) | Pooled Tissues (N=49) |
| Rosario | 0/25 | 0/15 |
| Padre Garcia | 0/14 | 0/14 |
| Lemery | 0/35 | 0/20 |
| Tanauan | 0/2 | No sample |
| Positive Rate | 0% | 0% |
-s are spread from LBM. According to Kim et al. (2012), LBM provides a venue for poultry viruses to be mixed and to be transmitted to new host, hence increasing the chance of viral evolution. Interspecies transmission of NDV can change the virulence of a low virulent strain in the LBM environment (Kim et al., 2012). In the study of poultry handling practices in LBMs conducted by Mulisa et al. (2014), they observed several practices that could promote ND transmission on the live birds. These include limited cleaning and disinfection of the area and holding cages, mixing of birds of different origin, disposable of sick and dead birds and poor proper waste disposable. Molia et al. (2015) identified risk factors and characteristics that were associated to the presence of ND and Avian influenza in LBMs such as being open every day with more than two days before a bird is sold, absence of zoning to segregate poultry related work flow areas, waste removal or cleaning and disinfecting less frequently than on a daily basis, trash disposal of dead birds and absence of manure processing.
It is highly evident from this study that the examined LBMs from Batangas, Philippines contain multi species of live birds of different ages that were being sold in streets and public places where high human and vehicle traffic occur. However compared to other LBMs from other countries, LBMs in Batangas operate very shortly and all birds are usually sold out within half the day. According to the vendors, disinfection and cleaning of LBM premises are also done on a daily basis. However, absence of zoning to segregate poultry related work flows was observed on the LBMs of Rosario, Padre Garcia and Lemery, Batangas. Direct contact with contaminated cages, food, water and feces and cross contamination among the different species of birds of different ages are highly possible. Considering that most of these birds are unvaccinated, these LBMs provide a conducive environment for the transmission of NDVs in the Philippines.
Serological analysis showed that sampled birds from the different LBMs in Batangas, Philippines had ND HI antibody titers of 22to 29. Out of 49 sera tested, 48 (97.96%) native chickens were seropositive for NDV. Based on records and questionnaires, all native chickens from the different LBMs were all unvaccinated against NDV. According to OIE (2017), HI antibody titer is regarded as positive if there is an inhibition at 1:16 dilution (24) or more using 4HA units as working antigen. Positive serology on unvaccinated birds may be considered as diagnostic evidence of ND and an indication that these birds have contracted the disease and recovered thereafter (Awan et al., 1994; Rezaeianzadeh et al., 2011) or have subclinical infection (Jibril et al., 2014; Eze and Ike, 2015). The high antibody titer of the native chickens may indicate that these birds may have been previously exposed to virulent NDVs. In the study of Escamillas (2001), 58 (52.0%) sera from unvaccinated free-range native chickens out of 150 serum samples had HI titer ranging from 22 to 28 which may suggest previous exposure to NDV. Other serological studies conducted locally related to HI test for detection of NDV antibody were done by Mandigma (2000) on brahminy kite (Haliastus indus intermedius) and Philippine serpent eagle (Holospilus spilornis); Diaz (2001) on tree sparrows (Passer montanus); Naguiat (2002) on local ducks; Matienzo (2002) on trapped marsh birds; and Lastica-Ternura et al. (2016) on captive raptors.The detected high antibody titer against NDV in this study may mean that natural infection could have occurred since all the chickens were unvaccinated. However, clinical signs of ND were not observed on the examined birds during the sample collection and necropsy. The main necropsy findings observed in the sampled chickens (11 birds) were multifocal tumorous lesion in the intestines, which may suggest potential visceral Marek’s disease, Lymphoid leukosis, reticuloendothelisios, intestinal parasitism or other neoplastic diseases. Seropositive birds but without clinical signs of ND maybe an indication that these birds have previously been infected by non-virulent strains or have survived outbreaks of a virulent NDV strains (Terefe et al., 2015).
There are no published studies available related to molecular detection of NDVs in LBMs in the Philippines. In this study, all the tissue samples from the 49 native chickens and the 76 swab samples from different LBMs were negative in nested RT-PCR. This may indicate that no active infection maybe ongoing on the sampled native chickens. Similar results were obtained by Lastica-Ternura et al. (2016) where the swab samples from the 42 captive raptors were negative in nested RT-PCR assay while some of the birds were highly positive in HI test. The result of the molecular and the serological test suggested that the raptors may have recovered from previous NDV infection and no active infection maybe occurring on the birds (Lastica-Ternura et al., 2016). A molecular and serological study of NDV in unvaccinated village chicken conducted by Rezaeianzadeh et al. (2011) showed negative result on RT-PCR but positive reaction on HI test. They concluded that the serological finding may imply a circulation of NDV on the native chickens in the area while molecularly, no virus was detected since there was no occurrence of active viral infection. In contrast if NDV was detected on the pooled oropharyngeal and cloacal swab, this may suggests that the birds are in the early or advanced stage of the disease since NDV is transmitted through aerosol and ingestion or the birds are on the shedding stage of the infection (Haque et al., 2010). Failure to detect NDV in the seropositive native chickens may also be associated to the timing of sample collection. It can be assumed that at the time of sample collection, the birds may have recovered fully from the disease and no longer shedding the virus. For shedding birds, an infected chicken can shed the virus in the feces in which the virus can survive for more than 8 weeks given the temperature of 40°C (Awan et al., 1994). This form of inanimate reservoir can also contribute to the spread of NDV.
In summary, serological and molecular analyses were performed to detect and characterize the presence of NDV in LBMs in Batangas, Philippines. Although nested RT-PCR did not detect any ongoing active infection nor viral shedding in any of the birds examined during the time of the study, unusually high HI antibody titers against NDV were detected in the non-vaccinated birds. These may imply that the birds may have been previously exposed to virulent NDVs but may have already recovered from clinical disease. Market practices and identified risk factors that may promote the potential spread of the disease such as the ones reported here must be continuously reviewed and improved so that the potential role of LBMs as reservoir of NDVs to the commercial poultry industry could be effectively reduced. Serological and molecular detection of other common poultry diseases of native chickens should also be performed. Other domestic avian species that are being sold in live bird markets should also be tested for the presence of NDVs and other pathogens. Regular monitoring through disease surveillance and physical inspection of LBMs in the country should be regularly implemented to decrease the risk of NDV transmission to the commercial poultry industry and prevent any future ND outbreak in the Philippines.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Hiromitsu Katoh and Mrs. Masako Katoh of PPQC Foundation for the technical and logistical support in the conduct of this study.
Conflict of interest
There is no conflict of interest in the conduct of this study.
Authors Contribution
All authors contributed equally in the study. DVU conceived and designed the research. GJOR conducted the sample collection and serological tests. DVU performed the molecular tests and data analysis. DVU and GJOR drafted the manuscript.
REFERENCES






