Advances in Animal and Veterinary Sciences
Research Article
Practical Application of Guar (Cyamopsis tetragonoloba L. Taub) Meal in Poultry Nutrition
Muhammad Saeed1*, Faiz Ul Hassan2,Qurban Ali Shah3, Muhammad Asif Arain3, Mohamed E. Abd El-Hack4, Mahmoud Alagawany4, Kuldeep Dhama5
1Department of Animal Nutrition, College of Animal Sciences and Technology, Northwest A&F University, Yangling, China, 712100; 2Institute of Animal and Dairy Sciences, Department of Animal Breeding and Genetics, University of Agriculture Faisalabad, Pakistan; 3Faculty of Veterinary and Animal Sciences, Lasbela University of Agriculture, Water and Marine Sciences, 3800, Uthal, Balochistan, Pakistan; 4Department of Poultry, Faculty of Agriculture, Zagazig University, 44511 Zagazig, Egypt; 5Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, 243 122, Uttar Pradesh, India.
Abstract | Guar is a very important legume crop in arid areas of Indian sub-continent. Over the world, India is known as the biggest producer of guar bean by contributing around 90% of the total production. Guar meal is defined as a guar gum by-product which has about 450 gram protein per kilogram. In spite having high protein content, guar meal is not known on a commercial scale but it has received substantial attention for animal feedstuffs. Trypsin inhibitors are the main anti-nutritive factor present in guar; however, these can be ameliorated with the uses of enzymes and heat treatment. Fluctuations in market prices of essential feed ingredients motivated poultry nutritionist to look for alternative valuable replacements for certain feeds. Based on the promising findings of guar meal inclusion in diets, it might be an acceptable feed ingredient in poultry feed. Guar meal can be fed up to 100 g/kg diet without adverse effects on poultry performance. Higher level of guar meal at150 g/kg can be detrimental for broiler and layer birds. Because of increasing the availability of guar beans, opportunities have been expanded for using guar meal as a partial alternative for soybean meal (SBM).The inclusion of guar meal in poultry diets canbe maximized by the addition of some feed additives such as β-mannanases. The present review article presents thatnutritional values of protein and amino acids of guar meal is similar to the SBM and seems to be an attractive feed ingredient in the poultry industry.
Keywords | Guar meal, Nutritional values, Anti-nutritional factor, Poultry nutrition, Soybean meal.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 31, 2017; Accepted | September 10, 2017; Published | November 15, 2017
*Correspondence | Muhammad Saeed, Department of Animal Nutrition, College of Animal Sciences and Technology, Northwest A&F University, Yangling, China, 712100; Email: muhammad.saeed@nwsuaf.edu.cn
Citation | Saeed M, Hassan FU, Shah QA, Arain MA, El-Hack MEA, Alagawany M, Dhama K (2017). Practical application of guar (cyamopsis tetragonoloba l. Taub) meal in poultry nutrition. Adv. Anim. Vet. Sci. 5(12): 491-499.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.12.491.499
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Saeed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Guar (Cyamopsis tetragonoloba) which belongs to Fabaceae (Leguminaceae) family is upright, a drought-tolerant, a coarse and annual summer legume crop being planted as food for the consumption of livestock and human as well. The major producers of guar are India, Pakistan, and USA. However, smaller quantities are also grown in Africa and Australia (Gillett, 1958; Undersander et al., 2006; Dinani et al., 2017). Poultry industry is facing challenges regarding the availability of cheaper and quality feed ingredients due to the ever-increasing competition of utilization of agriculture land for industrial purposes rather than crop production.This is resulting in reduced production of feed ingredients which is one of the major factors limiting the development and expansion of poultry industry (Emenalom, 2004; Sapkota et al., 2007). Nowadays, scientists are searching for alternate and cheaper sources of feedstuffs in order to broaden range of available ingredients for poultry feed industry (Annongu et al., 2010). Guar is also one of the best medicinal plants mainly grown in Indian subcontinent The crop is grown on a commercial scale for its high concentrations of galactomannan, commonly well-known as guar gum (Gendy et al., 2013), which is being used in textile, paper, pharmaceutical and mining industries (Vishwakarma et al., 2012; Kadian et al., 2013). Guar is a known plant in folklore and traditional medicine. It could be used as a digestive tonic, laxative, appetizer and cooling agent. It is also useful in dyspepsia and anorexia. Guar has very potent antisecretory, hypolipidemic, anti-ulcer, hypoglycemic, anti-hyperglycemic and cytoprotective impacts (Mukhtar et al., 2008). Hassan et al. (2010) defined guar meal as a by-product derived from guar beans during extraction of galactomannan (guar gum) containing saponins. These saponins have been investigated for antibacterial (Hassan et al., 2007), and anti protozoal (Mshvildadze et al., 2000) activities and can be used as a feed ingredient in poultry feed (Gutierrez et al., 2007; Dinani et al., 2010). Guar Meal (GM) is being considered a relatively cheap along with high protein meal source (33 to 60 % according to the type of fraction) (Salehpour et al., 2012). According to Conner (2002), GM could be produced as a secondary product from the manufacture of guar gum. Authors added that GM is a prime choice of alternate protein source in poultry feed, may offer potential opportunities to use GM as the least cost poultry feed. Compared to SBM, GM has 88% crude protein exists as a true protein. GM is rich in its content of arginine; since it is deficient in threonine, methionine, lysine, leucine, and isoleucine (Verma and McNab, 1984). Threonine is a very important as limiting amino acid (AA) in avian nutrition. Its deficiency causes weight loss, lack of appetite, and inhibits bone formation. It has been reported that GM can be incorporated up to 150 g/ kg in the chickendiets without affecting the performance at 42 days of age (Rao et al., 2014). Soybean meal is a quality protein source for poultry feed but its use in poultry feed is limited due to shortage and high cost. Therefore; nutritionists generally look for viable and cheaper alternate protein ingredients to replace SBM. Aforementioned literature shows that GM, as a feed ingredient, might be used in poultry nutrition to resolve this problem because amajor part of crude protein (88% CP) in GM is available as a true protein with high arginine content. Arginine plays a significant role in cellular physiology and metabolism in birds. This review article aims to broaden our understanding of nutritional value in terms of protein and AA profile of GM in comparison to SBM and seems to be an attractive replacement for SBM in poultry feed industry.
Trade and Global Production Of Guar
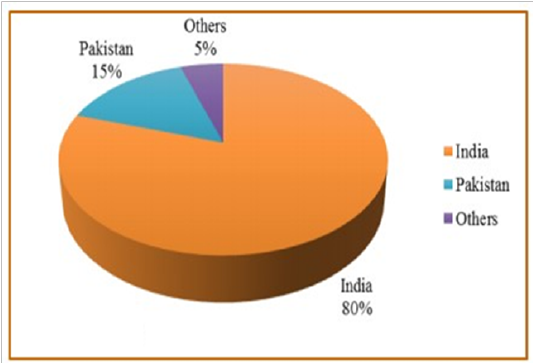
Annually, guar seed is globally produced by about 1.0 to 1.6 million tons. Since, monsoon conditions in India are one of the various reasons which mainly cause the extensive fluctuation in guar production (Sharma and Gummagolmath, 2012). Figure 1, shows that about 80% of the globalproduction of guar seed is produced from India, so it is considered as the biggest producer of guar and its derivatives around the world followed by Pakistan which shares by about 15%. Other contributing countries are Brazil, Zaire, China, Malawi, Sudan South Africa, Australia, and USA. Currently, there is a risein guar products prices, so China and Australia are supporting guar production and cultivation on a large scale area, to challenge India’s monopoly.
Nutrient Composition of Guar Meal
Different researchers have described nutritional contents of GM of dry matter (DM), ether extracts (EE), crude fiber (CF), CP, AA and mineral contents. Studies have reported that GM contains 92 to 96.7% DM (Ahmed, 1994; Lee et al., 2004; Nadeem et al., 2005). It was reported that ash contents in GM is around 4%, while other studies have reported 3.8% and 5.5% ash in guar seed and meal, respectively (Nagpal et al., 1971; NRMC, 1994). In another study, the ash content of different fractions of guar was reported as germ (6.8%), hull (5.7%) and combined guar by-products (5.4%) (Verma and McNab, 1982).However, variable ash contents have also been reported for the same fractions, respectively (Lee et al., 2004). Guar meal generally contains 35 to 47.5% CP but variable levels in different studies have also been reported (Couch et al., 1966; Ambegaokar et al., 1969). Like, in guar germ (30%), mechanically extracted (30%) and solvent extracted (41.3%) guar germ meal (NRMC,1994). Fairly higher CP contents like 45.9%, 36.9%, and 38.5% have been reported in guar germ, hull, and combined guar by-products, respectively (Lee et al., 2004). Recently, the percentage of DM, organic matter (OM), CP, EE, CF, nitrogen-free extract (NFE), ash, acid detergent fiber and neutral detergent fiber for guar meal were 93.56, 94.66, 49.52, 3.59, 4.46, 37.09, 5.35, 22.59 and 44.05 respectively, on DM basis (Janampet et al., 2016). Chemical compositions of GM and SBM are explained in Table 1.
Table 1: The chemical compositionof different guar meals in comparison with soybean meal.
| Parameters | Guar Meal Churi | Guar Meal Korma | Roasted Guar Meal Korma | Guar meal Microlam | Soybean meal |
| Protein (%) | 40 | 50 | 56-58 | 58-60 | 46-48 |
| Fiber (%) | 1 | 5-6 | 3-4 | 2-3 | 6 |
| Fat | 3-4% | 4-5% | 4-5% | 4% | 0.20-1% |
| Ash/sand-silica | 7% | 5% | 1-2% | 1% | 2% |
| Total energy | 2698 K Cal /Kg | 4050 K Cal /Kg | 4100 K Cal /Kg | 4250 K Cal /Kg | 3650 K Cal /Kg |
| Calcium | 0.30 | 0.55 | 0.55 | 0.55 | 0.25 |
| Phosphorus | 0.42 | 0.68 | 0.68 | 0.68 | 0.58 |
| Pepsin digestibility | 87-90% | 80% | 87% | >97% | 84% |
| Trypsin Inhibitor | 2mg/g | 2mg/g | <1mg/g (almost NIL) | <1mg/g (almost NIL) | >2mg/g |
| Urease Activity | <0.10% | <0.10% | <0.10% | <0.10% | <0.10% |
|
Aflatoxin |
- | - | - | - | - |
| Salmonella | - | - | - | - | - |
| E coli | - | - | - | - | - |
| Note | Partial substitute of soybean meal | Best substitute for soybean meal | Substitute of fish meal, potato meal, corn gluten meal |
Source: http://guarmeal.com/product/(date of access)
Amino Acids Profile of Guar Meal
Guar meal has very rich amino acid profile (Table 2) as it contains high lysine and arginine which is almost double as compared to SBM (Verma and McNab, 1982).
Table 2: Amino acid composition of guar meal korma in comparison with soybean meal
| Amino Acids (Units) (%) | Guar Korma | Soybean Meal |
| Arginine | 9.96 | 3.39 |
| Cystine | 0.70 | 1.00 |
| Histidine | 1.19 | 3.75 |
| Isoleucine | 2.03 | 1.45 |
| Leucine | 3.49 | 2.40 |
| Lysine | 2.85 | 2.75 |
| Methionine | 0.57 | 2.96 |
| Phenylalanine | 2.22 | 3.47 |
| Threonine | 1.78 | 0.03 |
| Tryptophan | 0.64 | 0.52 |
| Tyrosine | 1.57 | 6.13 |
| Glycine | 2 | 5.85 |
| Valine | 1.8 |
2.54 |
Source: http://guarmeal.com/product/
Anti-Nutritional Factors of Guar Meal
Various studies have documented the presence of anti-nutritional factors like saponin, β-mannan, and trypsin inhibitor in GM (Acamovic, 2001). Guar meal is considered a good protein source due to its high CP (35–45 %) along with an excellent blend of AA especially higher lysine and arginine content which make it a promising ingredient for poultry diets (Ahmed et al., 2006). However, guar bean contains some anti-nutritional factors like trypsin inhibitor and a galactomannan (guar gum) which affect feed conversion ratio and growth performance in broilers (Saxena and Pradhan, 1974). Diets supplemented with guar bean increases intestinal viscosity due to β-galactomannan leading to reduced nutrient digestibility especially in a day old chickens (Lee et al., 2003; Abu Hafsa et al., 2017). This increase in the intestinal viscosity has also a negative effect on passage rate resulting in poor feed intake (Yasar, 2003). Different processing techniques like heat treatment have been used to reduce emulsification and foaming in guar bean to increase its nutritional value (Nidhina and Muthukumar, 2015). The treatment with enzymes has also been reported to ameliorate growth-depressing effects of guar gum (Vohra and Kratzer, 1964). The quantities of antinutritional agents (trypsin inhibitors, phytate, and phenolic) in legume seeds have been observed to decrease during sprouting (Joshi and Varma, 2016) which ultimately improves the nutritional values (Sathithon and Yan-bin, 2012).
Methods To Improve The Nutritive Value Of Guar Meal
Nowadays various strategies like heat processing, heat/water treatment and enzyme supplementation were used to improve the nutritive value of GM by mitigating effects of anti-nutritional factors. Heat treatment and supplementation of enzymes like β-mannanase or hemicellulase alone or both, results in better feed utilization (Patel and McGinnis, 1985; Lee et al., 2003a; 2003b; 2005; 2009) (Table 3). Theenzymes like pectinase in chick feed have also been recommended for overcoming growth-inhibitory properties of GM (Anderson and Warnick, 1964; Rao et al., 2015).
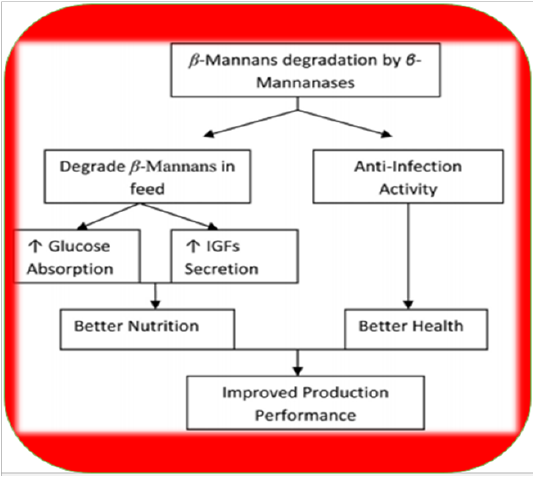
Fermentation of GM with Aspergillus niger or Fusarium has been shown to improve production performance in chicken (Nagra et al., 1998). Heat treatments also have negative effects like sticky droppings; however, supplementation of enzymes can avoid such issues (Patel and McGinnis, 1985). Moreover, it is recommended to keep inclusion level of GM below 2.5% to avoid these problems (Lee et al., 2003b). Nowadays toasting is practiced to remove unwanted chemicals (trypsin inhibitors, haemagglutinins, etc.) but still, other treatments are required to remove residual gum and ameliorate its adverse effects. The addition of enzymes in guar meal based diets can reduce the viscosity of intestinal digesta to overcome its detrimental effects in broilers (Burnett, 1966; Almirall et al., 1995; Ehsani and Torki, 2010). Supplementation of β-Mannanase in poultry diets containing guar meal has revealed beneficial impacts like lowered viscosity of intestinal digesta, better growth performance, improved FCR (Lee et al., 2003b; Ehsan, and Torki, 2010) optimum gut health and immune status (Mehri et al., 2010). Moreover, it enables the bird to fight Salmonella enteritis (Gutierrez et al., 2008). β-mannanase hydrolyzes β-1, 4-glycosidic bonds in β-mannans (Ooi and Kikuchi, 1995). Hydrolysis of guar gum galactomannan produces mannooligosaccharides which can improve growth performance of birds by inhibiting Salmonellae and E. coli colonization (Ishihara et al., 2000). It also decreases β-mannan levels in GI tract which increase stimulation of innate immune response as presented in Figure 2 (Jackson et al., 2004). The activation of the innate immune system is mediated by β-mannanase absorption leading to enhanced proliferation of macrophages and monocytes and cytokine synthesis. Many studies have shown positive effects of endo -β-D-mannanase enzyme on the nutrient utilization ofpoultry diets low in β-mannans (typical corn-soy-based diets) and other feed materials like as copra-meal and guar which are rich in mannan (Table 4).
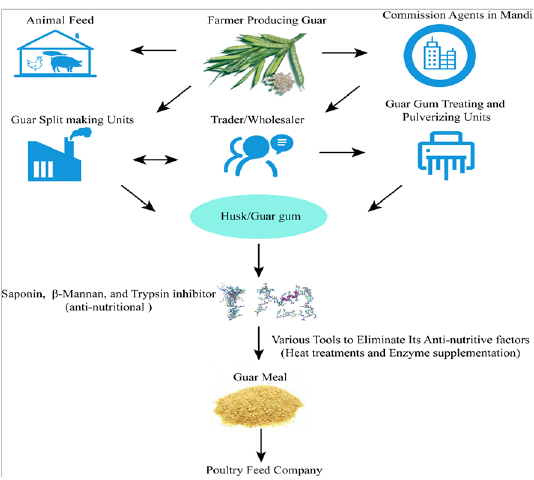
It is evident from the literature that β-mannanase supplementation of guar gum based diets can decrease the viscosity of digesta in poultry (Lee et al., 2003b) and improve utilization of nutrients (Ray et al., 1982; Verma and McNab, 1982). Broiler diets contained guar gum (2%) with enzyme addition significantly enhanced the gain of net energy and depressed DM and N in feces (Daskiran et al., 2004). Moreover, it was found that growth performance of broilers fed low-energy diets and enriched with β-mannanase was better than that of high-energy diets free of enzyme supplementation (McNaughton et al. 1998). This energy sparing effect was also reported by Maqbool et al. (2010) who observed that enzyme addition (like hemicellulose) to rations with GM (level) could spare about 90 Kcal/Kg ME. Moreover, Mussini et al. (2011) examined different dosages of β-mannanase (CTCzyme) (0%, 0.025%, 0.05%,0.1%) and found that excreta gross energy depressed with elevating the level of enzyme which led to more efficientutilization of nitrogen by the birds. The β-mannanase has also been reported to enhance the overall health of birds by influencing themorphology of gut and plasma immunological status in broilers (Christine et al., 2002; Mehri et al., 2010). Flow diagram depicting overall guar meal processing steps of feed mills (Figure 3).
Table 3: Tool to eliminate anti-nutritional factor (enzymes, source organism, substrate, their function and probable results).
| Name /classification | Source Organism | Substrate | Function | Probable results |
| Hemicellulase | Aspergillus aculeatus Aspergillus niger Bacillus lentus Bacillus subtilis Humicola insolens Trichoderma longibrachiatum | Milo, barley, rye, oats, wheat, corn, legumes | Breaks down hemicellulose | Reduced digesta viscosity; decreased anti-nutritional effects NSP by increasing solubility of NSP in GIT; increased hemicelluloses digestibility ultimately increasing the energy value of feed; reduction in stickiness dropping in poultry |
| β-Glucosidase | Aspergillus niger | Plants with oligosaccharides | Hydrolyzes cellulose degradation products to glucose | |
| β-Mannanase | Aspergillus niger, var. Bacillus lentus Bacillus subtitles Trichodermalongibrachiatum | Cereals and legumes (e.g. Guar meal) | Hydrolyzes β - mannans, a component of hemicellulose | Decreased anti-nutritive effects of β-mannans by reducing digest viscosity; increased β-mannans digestibility in feed, production of oligosaccharides and ultimately improved thevalue of feed (energy). Also decreased the problem of sticky dropping. |
(Adapted from Patel and McGinnis, 1985; Lee et al., 2003a; 2003b; 2005;2009)
Table 4: Summarized different trials that compiled the effect of guar gum, meal and β-mannanase on performance parameters
| Treatments (Guar gum %) | Week 1 | Week 2 | |||||
| BW (g) | Water:feed (g:g) | FCR | BW (g) | Water:feed (g:g) | FCR | Reference | |
| 0 | 178.6 | 2.673 | 1.019 | 392.5 | 2.347a | 1.261 | |
| 0.5 | 174.9 | 2.755 | 1.021 | 381.2 | 2.385a | 1.271 | |
| 1 | 170.8 | 3.031 | 1.032 | 383.1 | 2.478b |
1.306 |
|
| 2 | 153.8 | 3.043 | 1.16 | 344.8 | 2.386a | 1.506 | |
| Treatments | Feed consumption (g) | Weight gain | FCR | Mortality (%) | |||
| 0% guar meal | 3664.33ac | 1796.10a | 1.99a | 3.33 | |||
| 5% guar meal | 3736.20ba | 1723.90a | 2.14a | 0 | |||
| 10% guar meal | 3604.93c | 1538.44b | 2.33b | 3.33 | |||
| 15% guar meal | 3254.83d | 1265.90c | 2.56c | 6.66 | |||
| 0% guar meal | 3631.20ac | 1817.77a | 1.97a | 3.33 | |||
| + enzyme | |||||||
| 5% guar meal | 3797.16ba | 1813.60a | 2.00a | 0 | |||
Uses Of Guar Meal as a Feed Ingredient in Poultry Feed
Guar meal is an excellent essential amino acids source, with high levels of lysine, tryptophan, isoleucine valine and phenylalanine. The content of amino acids in guar meal makes it a useful protein source for layers and broilers (Lee et al., 2003). Guar meal is attained after mechanical separation of endosperm from hulls and germ of ground seeds. According to chemical analysis, it contained fat 4%; CF 6%; moisture12%; CP45%; and ash 4.5% and is considered a well- balanced protein source rich in lysine and sulfur-amino acids (Ramakrishnan, 1957).
Growth Performance and Carcass Characteristics
Various studies have been conducted to evaluate the use of GM at different inclusion levels in broiler diet. It has been reported that partially replacing SBM with GM not shown negative effects regarding, FCR, weight gain and carcass quality in chickens (ŞAraet al., 2015). Studies revealed that GM included at 2.5% and 5% in poultry feeds, had no negative effects on growth performance of broilers, however, higher inclusion levels (7.5 and 10%) would negatively influence growth performances and health (Lee et al., 2003). Similarly, replacing SBM with GM in broiler diets at levels of 3-18% at different growth stages, showed an improvement in growth performances and carcass parameters at lower levels of 3-9 % (Gheisari et al., 2011). Another study revealed optimum inclusion level at 50 g/ kg of sprouted guar than roasted guar bean meal suggested beneficial for growth in broiler diets (Madzimure et al., 2017). A study of Janampet et al. (2016) confirmed that the inclusion of 50% guar meal instead of groundnut cake enhanced the growth rate and digestion coefficient of nutrients without any adverse effects on performance. No significant difference was observed in the growth performance among guar meal groups. Similar results were stated by Goswami et al. (2012) and Jongwe et al. (2014), but these findings disagreed with the results of Salehpour and Qazvinian (2012) who found that decreased growth rate with increasing level of guar meal in poultry diet.
It has been observed that using GM up to 5% without enzyme or up to 10 % GM with Hemicell enzyme in diets showed no adverse effect on performance and carcass traits in broilers (Afrouzi et al., 2016). Guar meal has been proved as a cheaper feed ingredient than cotton seed cake (CSC) and replacing CSC with GM helped in economical ration formulation without any detrimental effects on growth performance in animals (Sharif et al., 2014). Furthermore, enzyme supplementation in GM diets has shown positive effects on dressing percentage in broilers (Ahmed et al., 2015). Studies involving a dietary inclusion of guar in different avian species are summarized in Table 5.
Table 5: Dietary inclusion of guar in different animal species.
| Animal Species | Dietary Inclusion |
| Poultry | 2.5 to 3.0% |
| Broiler | 2.5 to 3.0% |
| Laying hens | 4.5 to 5.0% |
| Duck | 10 to 12% |
| Quail | 18 to 20% |
| Cattle | 2.5 to 3.0% |
| Sheep | 2.5 to 3.0% |
| Pig | 2.5 to 3.0% |
| Rabbit | 18 to 20% |
| Fish | 18 to 20% |
Source: http://guarmeal.com/dosage/
Blood Biochemical Parameters
The diet containing GM at 10% revealed a significant decrease in glucose levels (Rainbird et al., 1984). Moreover, enzyme supplementation in GM diets has shown positive effects on serum high-density lipoprotein (HDL) in broilers (Ahmed et al., 2015). Supplementation of 15% GM with or without enzyme supplementation and 15% fermented toasted guar meal (FTGM) found to be most effective in reduction of serum cholesterol and glucose level. On the other hand, Bhutia (2006) stated that blood contents of albumin, protein, globulin and albumin to globulin ratio were not affected by the feeding of GM in broiler chickens and quails. In broiler quail, use of 15% toasted GM with or without enzyme and 15% FTGM in the diet found to be most effective in lowering cholesterol and glucose level in quail serum (Dinani et al., 2017).
Broilers fed GM diets improved serum antibody titer against New castle disease virus (NDV) at 21 and 42 days of age of chicken (Ahmed et al., 2015). In another study, the humoral immunologic response found that the antibody titer against IBV was significantly increased in broilers chicks fed with GM diets (Gharaei et al., 2012). Regarding the humoral immunity, sheep red blood cells improved (6.25±0.25) in 7.5% fermented toasted GM in comparison with the control (4.25±0.24) group. Moreover, the values of haemagglutination titer were significantly higher in FTGM at 7.5 and 15% as compared to control and other levels (Dinani et al., 2017).
CONCLUSION
Guar meal can be a potential feed ingredient for poultry feed owing to its higher true protein contents and better amino acid profile. Anti-nutritional factors of GM can be reduced by supplementing diets with β-mannanase enzymes that would be helpful to reduce its negative effects and help in improving growth performance of poultry. Diets containing lower levels of GM has shown benefits to improve feeding value of poultry feed (100 g/kg feed). Moreover, enzyme supplementation in GM diets has shown positive effects on lipid profile and immune function.
CONFLICT OF INTERESTS
There were no conflicts of interests.
ACKNOWLEDGMENTS
All the authors of the manuscript thank and acknowledge their respective Universities and Institutes.
AUTHORS’ CONTRIBUTION
All authors contributed equally to this manuscript.
REFERENCES