Advances in Animal and Veterinary Sciences
Research Article
Efficacy of Tulathramycin in the Treatment of Respiratory Pasteurollosis in Rabbits
Nagah E. Edrees1, Suhair A. Abdellatief1, Ahlam E. Abdellatief2, Amany O. El-Sharkawy2
1Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Sharkia, Egypt; 2Pharmacology Department, Animal Health Research Institute, Zagazig branch, Zagazig 44511, Sharkia, Egypt.
Abstract | Pasteurella multocida is the most critical respiratory bacterial infection of rabbits, around the globe. Beside passive immunization, anti-bacterial treatment is the primary choice of disease control. Since tulathromycin may be retained in the lung for many days after single administration, it can be applied for treatment of respiratory diseases. Hence, the purpose of current study was to evaluate the efficacy of tulathromycin in treating experimentally infected rabbits with P. multocida. In the sensitivity test, tulathromycin showed a potent inhibitory effect on P. multocida compared to several well-established antimicrobial agents. In vivo, treatment with tulathromycin improved clinical signs, mortality rate, lesion scores and growth performance parameters in infected rabbits. Furthermore, treatment with tulathromycin ameliorated the hematological picture, lowered the level of biochemical parameters which were significantly increased due to infection as liver enzymes, blood urea, creatinine and creatine kinase (CK-MB) and elevated the levels of total protein and albumin. Biochemical findings were supported by histopathological picture. It can be concluded that tulathromycin is an ideal and safe antibiotic for treating infection with Pasteurella multocida in rabbits.
Keywords | Tulathromycin, Pasteurella multocida, Lung, Liver, Kidney, Heart
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 26, 2017; Accepted | August 18, 2017; Published | November 05, 2017
*Correspondence | Suhair A. Abdellatief, Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Sharkia, Egypt; Email: s.abdellatief777@gmail.com
Citation | Edrees NE, Abdellatief SA, Abdellatief AE, El-Sharkawy AO (2017). Efficacy of tulathramycin in the treatment of respiratory pasteurollosis in rabbits. Adv. Anim. Vet. Sci. 5(12): 477-485.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.12.477.485
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Edrees et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pasteurellosis caused by Pasteurella multocida is one of the most significant bacterial diseases of rabbits and causes considerable losses in expansive production units through out the world (Takashima et al., 2001). Pasteurella is considered an opportunist or secondary pathogen and can be found in the respiratory tract of healthy and diseased animals (Dziva et al., 2008). It is the most widely studied member of the genus and is the causative agent of fowl cholera, hemorrhagic septicemia, and a variety of respiratory syndromes such as atrophic rhinitis of swine and purulent rhinitis of rabbits (snuffles) (Arumugam et al., 2011). Snuffles is a highly contagious respiratory disease, which causes high mortalities in rabbit farms. It can be transmitted by direct and indirect contact mainly by aerosol (Premalatha, 2009).
Infection with P. multocida is endemic; therefore, it remains a source of considerable potential loss. Control measures such as enhanced husbandry and the culling of symptomatic animals reduce overall morbidity and mortality, but impose significant costs. Vaccines targeting P. multocida reduce infection but do not give complete protection under field conditions (Dabo et al., 2008).
Tulathromycin is a semi-synthetic macrolide antibiotic affirmed for use in treating bovine and swine bacterial respiratory ailments (Nowakowski et al., 2004). It acts by inhibiting protein synthesis of bacteria by binding to bacterial 50s ribosomal subunits stimulating the dissociation of peptidyl-tRNA from the ribosome during the translocation process which leads to a disruption of bacterial protein synthesis (Evans, 2005). In addition to impacting enhanced tissue and cellular penetration characteristic of all macrolides, this novel structure (tulathromycin) possesses alluring antibacterial properties particularly against Gram negative respiratory bacteria (Brunton et al., 2008).
The scope of this study was to assess the in vitro and the in vivo efficacy of tulathromycin against Pasteurella multocida infection in rabbits.
MATERIALS AND METHODS
Chemicals
Tulathromycin (Draxxin®) was gotten from Pfizer (Pfizer Animal Health, NewYork, NY).All kits used in the current study were obtained from Biodiagnostics Co. (Cairo, Egypt). All other chemicals were of analytical grade.
Experimental Animals
Forty male New Zealand rabbits of 8-10 weeks old with range body weight 1500-1600 g were obtained from the animal house of the National Central Institute, Dokki, Cairo, Egypt. They were fed on a balanced commercial ration and water was provided ad libitum. They were battery-reared in separate units. All hygienic measures were supplied as recommended. Rabbits were accommodated to laboratory conditions for ten days before being experimentally used. The experimental protocol was conducted according to the Guide for Care and Use of Laboratory Animals Guidelines of the National Institutes of Health (NIH) and affirmed by the research ethical committee of the Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt.
Antimicrobial Assay Test Organisms
P. multocida was obtained from Animal Health Research Institute, EL-Dokki, Cairo. Colonies were suspended in sterile saline and the density was adjusted to contain 5×109 bacterial cell/ml.
Experimental Design
Rabbits were allocated into 4 groups, 10 rabbits for each. Group I: (control) orally given saline solution (0.025 ml/kg BW, S/C). Group II: (Non infected treated), received a single dose of tulathromycin (2.5 mg/kg BW, S/C) (Abo-El-Sooud and Abd-El-Aty, 2012). Group III: (Infected non treated) rabbits were experimentally infected with p. multocida 0.2 ml/rabbit of broth culture contain 5×109 cell/ml, subcutaneously (Nassar et al., 2013). Group IV: (Infected treated) infected rabbits were injected with tulathromycin (2.5 mg/kg BW S/C) as a single dose. Treatment started after appearance of clinical sings. Rabbits were daily observed. Clinical signs, feed consumption, body weight, mortalities and lesion scores were recorded.
Re Isolation of Microoganisms
P. multocida was isolated from infected rabbit blood, cultivated on blood a gar plates and incubated for 24 hours at 37°C (Dziva et al., 2008; Jabeen et al., 2013).
Sensitivity Test
The disc diffusion technique was performed according to Greenwood, (1978).
Sampling
Two blood samples were gathered from ear vein at 3 days post infection, 3 and 10 days post treatment. The 1st sample was taken on EDTA for hematological studies and the 2ndone was collected without anticoagulant and left to clot then centrifuged at 3000 rpm for 10 minutes to get clear serum which was kept at -20 oC till be used.
Lung, liver, kidney and heart samples were fixed in 10% neutral buffered formalin for histopathological examination.
Hematological Findings
Hematological parameters (red blood cells (RBCs), hematocrit (PCV), hemoglobin (Hb), total and differential leukocytic count) were determined utilizing automatic cell counter.
Biochemical Analysis
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined (Tietz, 1976). Alkaline phosphatase (ALP) was estimated (Belfield and Glodberg, 1971).Activity of Gamma glutamyl transfer as (GGT) was assessed (Kaplan and Pesce, 1992). Serum total protein and albumin were detected according to Henry, (1974) and Doumas and Biggs, (1976) respectively. Globulin concentrations were determined by subtracting the concentration of total protein from albumin concentration. Serum urea and creatinine were evaluated (Vassault et al., 1986; Henry, 1974). Creatine Kinase-MB (CK-MB) activity was determined according to Gerhardt and waldenstran, (1979).
Histopathological Examination
Tissue samples were collected and fixed prior to routine processing in paraffin wax. Sections (5μm thick) were cut and stained using Hematoxylin & Eosin (H&E) and examined microscopically (Bancroft and Stevens, 1996).
STATISTICAL ANALYSIS
Statistical analysis was carried out using student’s T-test which was done according to Snedecore and Cochran, (1982).
RESULTS AND DISCUSSION
In Vitro Studies Re-Isolation of Pasteurella Multocida
Round, gray and mucoid colonies with sweetish odour were observed on blood agar. Similar findings were early noted by Dziva et al. (2008) and Jabeen et al. (2013).
Sensitivity Test
Tulathromycin revealed a more potent inhibitory effect on tested P. multocida than other tested antimicrobial agents, (Table 1). Similar in vitro study approved tulathromycin efficacy against P. multocida (Clothier et al., 2012).
Table 1: Sensitivity of pasteurella multocida to tulathromycin and other antimicrobials (disc-diffusion method).
|
Chemotheraputic disc |
Concentration of disc (ug) |
Inhibition zonediameters (mm) |
| Tulathromycin | 30 | 25 |
| Gentamycin | 10 | 15 |
| Tetracycline | 30 | 10 |
| Ciprofloxacin | 5 | 12 |
| Lincospectin | 109 | 8 |
In Vivo Studies Clinical Signs
One day after challenge with P.multocida, infected rabbits showed depression, off food, snuffles, which manifested by mucopurulant nasal discharge, sneezing, gasping, mouth breathing, conjunctivitis with serous purulent exudates, abscess formation, neurological signs with rolling, weight loss and death. Infected group treated with tulathromycin showed milder symptoms 3 days post treatment which nearly disappeared 10 days post treatment. Similar manifestations observed by Nassar et al. (2013).
Feed Intake, Live Body Weight and Weight Gain
There was a significant (p < 0.05) increase in feed intake, body weight and weight gain of group II when compared with control group, (Table 2). These results endorsed those obtained by Enrico et al. (2016). Group (III) showed a significant decrease in feed intake, body weight (p <0.001) and weight gain (p <0.01) all over the experimental period when compared with control group. The higher body temperature caused by infection may prompt to decrease in feed intake and consequently body weight (Kulcsar et al., 2008). Treatment of infected rabbits with tulathromycin induced a significant (p < 0.01) increase in these parameters when compared with infected non treated group. This improvement may be due to the effect of tulathromycin on the bacterial infection. Our results clearly reinforced with those previously obtained by Kilgore et al. (2005).
Mortality Rate and Lesion Scores
Mortality rate in groups (I) and (II) was zero% and no lesions were detected in different organs, (Table 3). It was 50 % in group (III) which showed pathological lesions as pneumonia (90%), hepatitis (80%), pericarditis (50%), nephritis (50%) and subcutaneous abscess (20%). These lesions may be due to bacterial toxins, bacteremia and septicemia. The current data were supported by those reported by Nassar et al. (2013). Treatment of infected rabbits with tulathromycin reduced mortality rate to be zero% and gross lesions were pneumonia (20%), hepatitis (10%), pericarditis (zero), nephritis (zero) and subcutaneous abscess (zero).
Hematological Picture
A significant (p<0.05) increase in Hb concentration was noted in group II when compared with control group, (Table 4). Group III revealed a significant diminishing in Hb concentration (p<0.001), RBCs count (p <0.01) and PCV% when compared with control group. These effects may be due to the petechial hemorrhage present in different organs and or the effect of P. multocida endotoxins on RBCs which led to decrease of their life span. Walton, (2013) attributed anemia observed during infection to the shifting of iron to storage in the mononuclear phagocytic system and utilization of iron by bacteria makes it less available as erythroid precursors. Our outcomes were in the same direction with Praveena et al. (2007).Treatment with tulathromycin evoked a significant increment in the above mentioned parameters.
A significant (p <0.05) increase in neutrophils count and a noteworthy (p <0.01) decrease in lymphocytes count was observed in group II when compared with control group 3 days post treatment, (Table 4). Group III demonstrated a significant increase in total leucocytes (p <0.001) and neutrophils count, with a significant (p <0.05) diminish in lymphocytic count when contrasted and control group 10 days post treatment. These findings may be attributed to inflammation and endogenous steroids release as a response to infection (Ali et al., 2015). Similar results obtained by Vestweber et al. (1999) and Praveena et al. (2010). Treatment of infected rabbits with tulathromycin elicited a significant decline in total leucocytes and neutrophils count.
Biochemical Investigation Liver Function Parameters
The activities of ALT, AST, ALP and GGT were significantly increased in group (II) 3 days post treatment when compared with control group. Meanwhile, no significant changes were detected 10 days post treatment (Table 5). A significant (p <0.05) increase in the activities liver enzymes was observed all over the experimental period in group III when contrasted and control group. Infection produced alteration in cellular permeability due to changes in cell me-
Table 2: Effect of tulathromycin on body weight, body weight gain and feed intake of control and infected rabbits
| Parameters | Group | ||||
| Day of treatment | I | II | III | IV | |
|
Body weight (gm)
|
At first 0 3 10 |
1606.66±5.52 1703.33±4.62 1916.67±5.77 2286.66 ±6.01 |
1670.00±3.22 1786±3.13 2190.00±5.35* 2610.00±3.05* |
1610.00±3.94 1483.34±2.18** 1310.00±2.16*** 1050.00±2.02*** |
1620±2.59 1480.00±2.23 1766.66±2.10•• 2243.33±1.78••• |
|
Weight gain (gm) |
0 3 10 |
96.67±0.99 213.33±1.74 370.00±1.91 |
116±1.03 404.00*±1.70 420.00*±1.14 |
-126.66±0.69** -173.33±0.93** -260.00±1.11** |
-140.00±1.01 286.66±0.96•• 476.67±1.49•• |
|
Feed intake (gm) |
0 3 10 |
600.00±2.51 700.00±1.78 1233.33±3.64 |
633.33±3.09 850.00±2.01 1316.67±2.22 |
433.34±2.20 266.66±1.37** 200.00±0.89*** |
483.33±1.69 666.66±1.58•• 1016.66±1.49••• |
Data are expressed as means ± SE of 10 rabbits; * P ≤ 0.05 ** P ≤ 0.01 *** P ≤ 0.001 * Compared with group I; • P ≤ 0.05 •• P ≤ 0.01 ••• P ≤ 0.001 • Compared with group III.
Group I: (control) orally given saline solution (0.025 ml/kg b.wt, s/c). Group II: non infected and treated subcutaneously with a single dose of tulathromycin (2.5 mg/kg b.wt). Group III: (Infected non treated) infected with Pasteurella multocida 0.2 ml/rabbit of broth culture contain 5×109 bacterial cell/ml S/C. Group IV: infected rabbits treated with tulathromycin (2.5 mg/kg b.wt, S/C) as a single dose.
Table 3: Effect of tulathromycin on mortality rate and lesion scores of control and infected rabbits.
| Parameters | Groups | |||
| I | II | III | IV | |
| Rabbits No | 10 | 10 | 10 | 10 |
| Mortality rate (%) | 0 | 0 | 50 | 0 |
|
Lesion scores (%) Pneumonia Hepatitis Pericarditis Nephritis S/C abcesses |
0 0 0 0 0 |
0 0 0 0 0 |
90 80 50 50 20 |
20 10 0 0 0 |
Group I: (control) orally given saline solution (0.025 ml/kg b.wt, s/c). Group II: non infected and treated subcutaneously with a single dose of tulathromycin (2.5 mg/kg b.wt). Group III: (Infected non treated) infected with Pasteurella multocida 0.2 ml/rabbit of broth culture contain 5×109 bacterial cell/ml S/C. Group IV: infected rabbits treated with tulathromycin (2.5 mg/kg b.wt, S/C) as a single dose.
mbrane which permits the escape of these enzymes into serum in abnormal high level (Joan and Pannel, 1981). Comparative findings were recorded by Kamal, (2008) and Seleim et al. (2003).Treatment of infected group with tulathromycin induced a significant decrease in ALT (p <0.01) and AST (p <0.05) activities 3 days post treatment and a significant (p <0.01) decrease in ALT, AST, ALP and GGT activities 10 days post treatment when compared with group III.
On correlation with the control group, group II showed a significant (p <0.05) increase in serum total protein, albumin and globulin 3 days post treatment which disappeared 10 days post treatment (Table 5). There was a significant decrease in total protein and albumin with a significant increase in globulin of P. multocida infected non treated group. Both hypoproteinemia and hypoalbuminemia noted after infection with P. multocida may be ascribed to the state of anorexia and inability of the liver to synthesize proteins (Lee-lewandrowski and Lewandrowski, 1994). Our data were supported by Faez et al. (2013). Hyperglobulinemia, resulted from infection may be a response to immune defense. Similar preceptions were recorded by Coles, (1986). Amelioration of liver function parameters through the experimental period until regained their control levels after treatment with tulathromycin indicated its effectiveness in treatment of P. multocida infection in rabbits.
Kidney Function Parameters
Group (II) showed a significant (p <0.05) increase in creatinine level 3 days post treatment with no significant changes 10 days post treatment when compared with control group (Table 5). Infection with P. multocida resulted in a noteworthy (p <0.01) increment when compared with group (I). This may be attributed to the renal damage caused by the bacterial infection which leads to decrease of filtration rate of the kidneys and thus retention of blood creatinine and urea (Harvey, 1997). Moreover, high protein catabolism, impaired cardiac function and low renal blood flow (Radosistis et al., 2000). Similar effects were observed by Seleim et al. (2003). Treatment with tulathromycin produced a significant decrease in serum urea and creatinine as a result of improving of kidney function.
Serum Cardiac Injury Marker
On comparison to group (I), group (II) demonstrated a significant (p <0.01) increase in CK-MB 3 days post treatment and no significant change 10 days post treatment, (Table 5). P. multocida infected group showed a noteworthy increase in CK-MB level throughout the experimental pe-
Table 4: Effect of tulathromycin on erythrogram and leukogram of control and infected rabbits
| Parameters | Groups | ||||
| Day of treatment | I | II | III | IV | |
|
Hb (g/dl) |
0 3 10 |
12.05±0.03 11.96±0.07 12.13±0.09 |
12.03±0.14 13.8±0.08* 16.27±0.07* |
8.36±0.007** 6.9±0.007** 5.47±0.006*** |
8.7±0.01 10.7±0.005• 11.57±0.004•• |
|
RBCs (1012/l)
|
0 3 10 |
7.10±0.009 6.94±0.008 7.26±0.01 |
7.08±0.01 6.68±0.005 10.27±0.09 |
4.34±0.005** 3.3±0.004** 3.05±0.005** |
4.6±0.006 5.07±0.003• 6.33±0.003•• |
|
PCV (%) |
0 3 10 |
36.5±0.78 37.67±0.96 35.33±0.86 |
36.33±0.44 39.66±0.45* 44.66±0.78 |
28.67±0.65* 23.33±0.81* 23.00±0.75** |
26.67±0.58 35.00±0.91 36.67±0.51• |
|
WBCs (109/l) |
0 3 10 |
11.02±0.17 10.87±0.32 11.18±0.23 |
11.16±0.21 9.57±0.01 10.5±0.21 |
18.01±0.11** 21.32±0.16** 24.5±0.09*** |
22.2±0.92 18.22±0.11• 11.03±0.05•• |
|
Neutrophils (109/l) |
0 3 10 |
3.8±0.07 3.9±0.05 3.79±0.06 |
3.5±0. 08 4.8±0.06* 5.57±0.09 |
8.6±0.05* 11.57±0.03** 17±0.04** |
11.09±0.06 8.7±0.02 5.29±0.06• |
|
Lymphocytes (109/l) |
0 3 10 |
6.36±0.03 6.18±0.01 6.54±0.10 |
7.11±0.04 4.15±0.005** 4.14±0.01 |
7.6±0.06 7.89±0.01 3.84±0.007* |
9.37±0.09 7.17±0.05 4.98±0.01 |
Data are expressed as means ± SE of 10 rabbits. * P ≤ 0.05 ** P ≤ 0.01 *** P ≤ 0.001 * Compared with group I; • P ≤ 0.05 •• P ≤ 0.01 ••• P ≤ 0.001 • Compared with group III. Group I: (control) orally given saline solution (0.025 ml/kg b.wt, s/c). Group II: non infected and treated subcutaneously with a single dose of tulathromycin (2.5 mg/kg b.wt). Group III: (Infected non treated) infected with Pasteurella multocida 0.2 ml/rabbit of broth culture contain 5×109 bacterial cell/ml S/C. Group IV: infected rabbits treated with tulathromycin (2.5 mg/kg b.wt, S/C) as a single dose.
riod. The increased oxidative stress and depressed antioxidant status have deleterious effects on both cardiac structure and function. Also, bacterial endotoxins cause myocardial damage (Singal et al., 1998). Meanwhile, a significant (p <0.05) decrease in CK-MB was noted in group (IV) when contrasted and group (III).
The consequences of the current study revealed that administration of tulathromycin in healthy rabbits resulted in a temporary increase in serum levels of liver enzymes, total protein, albumin, creatinine and CK-MB 3 days post treatment then returned to their normal values at 10 days post treatment. Er et al. (2011a; b) reported that the significant changes in routine serum biochemical and hematological parameters created by tulathromycin were within normal limits.
Histopathological Findings
After treatment with tulathromycin, lung of group II showed slight congestion of thickened pulmonary blood vessels, (Figure 1a), group III showed pneumonic area represented by thickening of the interalveolar septa by extensive leukocytic infiltration, (Figure 1b), peribronchial lymphoid hyperplasia, (Figure. 1c) and severe vascular congestion, (Figure. 1d). Lung of group IV showed vascular congestion with perivascular leukocytic aggregation, (Figure 1e). Similar findings early observed by Nassar et al. (2013).
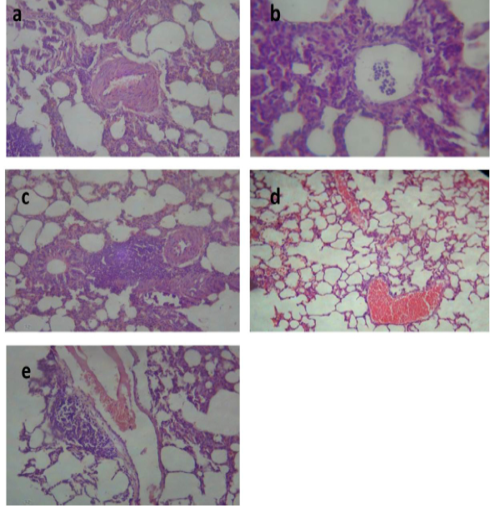
Figure 1: Lung of group (II) showing slight congestion of thickened pulmonary blood vessels (a) (H&E ×520). Lung of group (III) showing pneumonic area represented by thickening of the interalveolar septa by extensive leukocytic infiltration mostly polymorphonuclears (b) (H&E ×400), peribronchial lymphoid hyperplasia (c) and severe vascular congestion (d) (H&E ×100). Lung of group (IV) showing vascular congestion with perivascular leukocytic aggregation (e) (H&E ×100).
Table 5: Effect of tulathromycin on some biochemical parameters of control and infected rabbits
| Parameters | Groups | ||||
| Day of treatment | I | II | III | IV | |
| AST (U/L) |
0 3 10 |
53.73 ± 0.65 55.96±0.63 51.50±0.68 |
55.66 ± 0.77 89.34±0.59* 59.66±0.64 |
88.67 ± 0.61* 93.00±0.62* 125.30±0.65** |
91.33 ± 0.97 53.30±0.55• 53.70±0.54•• |
| ALT (U/L) |
0 3 10 |
39.83±0.69 38.00±0.67 41.60±0.78 |
38.67±0.42 64.66±0.50** 45.00±0.53 |
68.66±0.66* 110.00±0.59** 120.33±0.62** |
75.50±0.54 63.34±0.42•• 45.50±0.59•• |
|
ALP (U/L)
|
0 3 10 |
48.36±0.87 49.13±0.81 47.60±0.80 |
46.30±0.63 81.68±0.77* 53.61±0.61 |
84.66±0.85* 90.00±0.79* 122.65±0.72** |
104±1.18 88.40±0.89 62.40±0.57•• |
| GGT (U/L) |
0 3 10 |
4.4±0.08 4.1±0.06 3.9±0.07 |
4.09±0.01 7.1±0.01* 4.7±0.006 |
6.7±0.02*** 8.6±0.009 9.9±0.01** |
6.1±0.04 5.19±0.007 4.29±0.005•• |
|
Total protein (g/dl)
|
0 3 10 |
7.1±0.31 6.8±0.61 7.4±0.34 |
7.20±0.39 11.3±0.53* 9.4±0.65 |
5.7±0.28* 4.5±0.18** 5.1±0.22** |
5.8±0.24 6.1±0.17• 6.9±0.19• |
|
Albumin (g/dl)
|
0 3 10 |
4.7±0.22 4.5±0.28 5.3±0.27 |
4.4±0.19 6.4±0.27* 5.9±0.29 |
2.5±0.20* 1.8±0.21* 1.5±0.18** |
2.3±0.22 3.3±0.17• 4.7±0.11•• |
|
Globulin (g/dl)
|
0 3 10 |
2.4±0.25 2.3±0.21 2.1±0.22 |
2.8±0.10 4.9±0. 25* 3.5±0.20 |
3.2±0.17* 2.7±0.12** 3.6±0.15** |
3.5±0.21 2.8±0.09 2.2±0.06• |
|
Blood urea (mg/dl) |
0 3 10 |
23.5±0.44 22.66±0.39 24.33±0.41 |
22.67±0.33 24.00±0.40 25.67±0.34 |
38.34±0.37** 53.33±0.35** 54.67±0.32** |
40.33±0.56 27.33±0.31• 25.67±0.27•• |
|
Creatinine (mg/dl) |
0 3 10 |
1.12±0.06 1.00±0.05 1.25±0.07 |
1.17±0.02 2.34±0.03* 0.97±0.01 |
2.1±0.03** 2.3±0.02** 3.4±0.05** |
2.03±0.08 2.6±0.07 1.7±0.04• |
|
CK-MB (U/L) |
0 3 10 |
135.17±1.22 131.33±1.28 139.00±1.19 |
133.0±1.15 220.3±1.01** 156±0.81 |
227.3±1.07** 266.0±0.94** 317±0.72*** |
219.33±1.27 170±0.87• 148.66±0.65•• |
D Data are expressed as means ± SE of 10 rabbits. * P ≤ 0.05 ** P ≤ 0.01 *** P ≤ 0.001 * Compared with group I; •P ≤ 0.05 •• P ≤ 0.01 ••• P ≤ 0.001 • Compared with group III.
Group I: (control) orally given saline solution (0.025 ml/kg b.wt, s/c). Group II: non infected and treated subcutaneously with a single dose of tulathromycin (2.5 mg/kg b.wt). Group III: (Infected non treated) infected with Pasteurella multocida 0.2 ml/rabbit of broth culture contain 5×109 bacterial cell/ml S/C. Group IV: infected rabbits treated with tulathromycin (2.5 mg/kg b.wt, S/C) as a single dose.
Liver of group II revealed congested central vein and dilated hepatic sinusoids, (Figure 2a). Liver of group III showed severe congestion of the central veins, (Figure 2b). Cholangitis represented thickening of the bile duct wall by vascular congestion, edema and leukocytic infiltration besides cholestasis, (Figure 2c). Vacuolation of hepatocytes was also noted, (Figure 2d). In the liver of group IV, hepatic parenchyma showed residual hemorrhage but normal tissue articulation, (Figure 2e). Those outcomes affirmed the results of biochemical parameters and were in harmony with those of Dwivedi and Charan, (2001).
Kidneys of group II demonstrated focal leukocytic aggregation in the renal medulla, (Figure 3a). In kidneys of group III, vascular congestion and hemorrhage (Figure 3b) with fibrous connective tissue proliferation (Figure 3c) were observed. Meanwhile, kidney of group IV showed cystic dilatation of few renal tubules, renal casts, hydropic degeneration of the epithelial lining of some renal tubules and vascular congestion, (Figure 3d). These changes correlated with biochemical analysis of kidney function and were in concurrence with those reported by Palócz et al. (2014).
Histological picture of heart of group II revealed mild congestion of cardiac blood vessels, (Figure 4a). Heart of group III showed sever vascular congestion (Figure 4b), leukocytic infiltration between the myocardiocytes (Figure 4c) and inter muscular edema (Figure 4d). Those pathological findings were in agreement with those reported by Palócz et al. (2014). In group IV, heart showed very mild congestion of blood vessels with normal architecture of heart muscles (Figure 4e).

Figure 2: Liver of group (II) showing congested central vein and dilated hepatic sinusoids (a) (H&E ×400). Liver of group (III) showing severe congestion of the central veins (b). Cholangitis represented thickening of the bile duct wall by vascular congestion, edema and leukocytic infiltration besides cholestasis (c) (H&E ×100).Vacuolation of the hepatocytes (d) (H&E ×400).Liver of group (IV) showing interstitial hemorrhage and normal tissue architecture (e) (H&E ×520).

Figure 3: Kidney of group (II) showing focal leukocytic aggregation in the renal medulla (a) (H&E ×400). kidney of group (III) showing vascular congestion and hemorrhage (b) (H&E ×400) with fibrous connective tissue proliferation (c) (H&E ×100). Kidney of group (IV) showing cystic dilatation of few renal tubules, renal casts, hydropic degeneration of the epithelial lining of some renal tubules and vascular congestion (d) (H&E ×400).
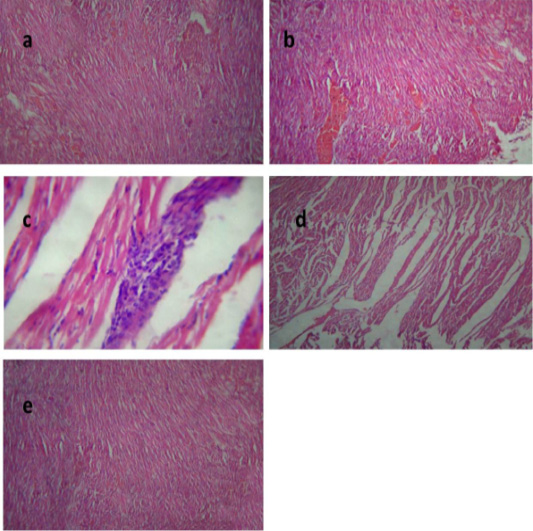
Figure 4: Heart of group (II) showing mild congestion of cardiac blood vessels (a) (H&E ×400). Heart of group (III) showing sever vascular congestion (b), leukocytic infiltration between the myocardiocytes (c) and intermuscular edema (d) (H&E ×100). Heart of group (IV) showing mild congestion of cardiac blood vessels (e) (H&E ×400).
CONCLUSIONS
In perspective of the findings detailed in this work, we may propose that tulathromycin as a single injection (2.5 mg/kg BW, S/C) was efficient against P. multocida infection in rabbits. This conclusion was clear from the decline of mortality rate and improvement of body weight, feed conversion ratio, blood picture, biochemical and histopathological picture.
Acknowlegements
This study received no support from any funding organizations.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Authors Contribution
Nagah E. Edrees and Ahlam E. Abd El-Latief carried out the experimental design. Suhair A. Abdellatief helped in performing the experiment and wrote the paper. Amany O. El-Sharkawy performed the experiment and the data analysis.
REFERENCES





