Advances in Animal and Veterinary Sciences
Research Article
Diagnostic Approach to Nephrotoxicosis with Vitamin K3 in Draft Horses Based on iNOS and Selective Urinary Variables
Wafaa A.M. Mohamed1, Yasmin H. Bayomi*2, Noura E. Attia2, Mustafa Abd El Raouf3
1Department of Clinical pathology, 2Department of Internal Medicine, 3Department of Surgery, Anaesthesia and Radiology; Faculty of Veterinary Medicine, Zagazig University, Egypt.
Abstract | A total of 17 draft horses were used in this study as two groups; vitamin K3 exposed group includes 7 draft horses administered vitamin K3 orally (4 field cases and 3 experimental cases).10 apparently healthy draft horses used as a control group. The four field cases showed signs of nephrotoxicosis 12-24 h following oral supplementation with double doses of vitamin K3(10mg/kg b.wt) with a one-day interval. The three experimental cases were administered vitamin K3 simulator to the field cases. The diagnosis was based on owner complaint, case history, duration of clinical signs, physical and clinical examination as well as ultra sonographical imaging, clinicopathological and histopathological findings. Results revealed that most of the vitamin K3 exposed group showed signs of renal colic, poor performance, depression, stranguria and oliguria. Laboratory findings showed hematuria, proteinuria, azotemia and hyperal -buminemia. Additionally, elevated urinary malondial dehyde, nitric oxide, gamma-glutamyl transferase, renal iNOS activities over baseline and declining in CrCl were observed. Histopathologically, proliferative glomerulonephritis and globular eosinophilic proteinisuos casts in the majority of the intact renal tubules were detected. In conclusion, oral vitamin K3 administration in equine resulted in oxidative and nitrosative renal damage and inducing acute renal injury, via increasing nitric oxide level and lowering the activity of nitric oxide synthase enzyme in renal tissue.
Keywords | ARI, GGT, Ca++ Carbonate, Hematuria, Renal colic, Nephrotoxicosis Vitamin k3
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 01, 2017; Accepted | October 25, 2017; Published | October 30, 2017
*Correspondence | Yasmin H Bayomi, Department of Internal Medicine, Zagazig University, Egypt; Email: yasmin.dream@yahoo.com
Citation | Mohamed WAM, Bayomi YH, Attia NE, El Raouf MA (2017). Diagnostic approach to nephrotoxicosis with vitamin k3 in draft horses based on inos and selective urinary variables. Adv. Anim. Vet. Sci. 5(11): 468-476.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.11.468.476
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Generally, acute renal injury (ARI) is a disease condition characterized by reduced glomerular filtration rate. It resulted from renal perfusion impairment (pre-renal cause), kidney dysfunction (renal cause), and urinary tract obstruction (post-renal cause) (Lameire et al., 2005). Animals with ARI clinically showed signs of acute colic, anorexia, laminitis, ataxia, mental depression, azotemia, oliguria, hematuria, proteinuria, glucosuria and crystalluria (Geor, 2007). In horses, the pre-renal causes of ARI include hypovolemia, severe diarrhea, excessive sweating, acute hemorrhage and disseminated intravascular coagulation (DIC) (Geor, 2003).The renal causes of ARI are ischemic necrosis of persistent renal hypoperfusion, interstitial nephritis and glomerulonephritis secondary to bacterial infections. While, the nephrotoxic agents include gentamicin, heavy metals, pigmenturia (hemoglobinuria and myoglobinuria), nonsteroidal anti-inflammatory drugs and hyper vitaminosis D and K3 (Bartol et al., 2000; Schrier and Wang, 2004).
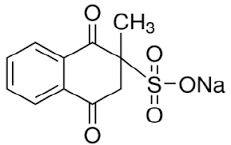
Although, vitamin K3 (menadione sodium bisulfite [C11H9NaO5S]) is an essential vitamin for bone health in horses, because it is the only vitamin K homolog has been found to increase the plasma concentrations of menaquinone-4 which poorly absorbed when itself administered, it has been largely thought to be an innocuous drug in horses (Terachi et al., 2011). It has a great role in bone repair in horses by increasing the equine bone density and decreasing breakdown. Inspite the former, it is reported as a potential candidate of ARI which was sufficient reason to be withdrawn from the US pharmaceutical markets (Rebhun et al., 1984). Its nephrotoxic impact was evident on the affected horse within 6 to 48 hr from its intravenous or intramuscular injection with a concentration of 2.2 to 11 mg/kg b.wt (Rebhun et al., 1984; Maxie et al., 1992). The associated signs include anorexia, depression, colic, hematuria, azotemia, stranguria, proteinuria, electrolytes imbalance, and isosthenuria as a result of acute tubular necrosis, interstitial fibrosis or chronic renal failure (Geor, 2007).
Ordinary laboratory diagnosis of renal function is warranted and mostly applied in horses. The new strategies for evaluation of vitamin K3-nephrotoxicosis pathophysiology in horses are still limited or nearly absent. In an attempt to follow the cause of vitamin K3-nephrotoxicosis in horses, biochemical analysis of inducible nitric oxide synthase (iNOS) enzyme of the affected horses was carried out as it is an essential enzyme responsible for synthesis of nitric oxide (NO) molecule which important in renal functions regulation (Mazroa et al., 2009). It produced by various endotoxins, cytokines, macrophages and vascular smooth muscle (Hibbs et al., 1988).Thus, the present work was established to follow the mechanism by which vitamin K3 induce nephrotoxicosis in horses using ordinary and semi-advanced technologies starting from clinical examination, hemogram, leukogram, serum and urinary variables till ultrasonography and histopathology.
Material and methodS
Tested Compounds and Reagents
Menadione sodium bisulfate,other chemicals and reagents were obtained from Sigma, St. Louis, MO plus El-Gomhouria Co., Egypt (GOMAC) for medical appliances and chemicals trading.
Study Description
The problem began when a veterinarian prescribed oral vitamin K3 to a horse, which suffered a fracture of the radius, with an injury that bleeding constantly. The lesion occurred accidentally by kicking from another stallion. The veterinarian point of view was to increase bone density, stop bleeding, improve healing and that oral administration might be not harmful as the injection. When the owner knows the benefits of vitamin K3, he decided to administer it for all his own horses (4 cases). After 12-24 h from oral administration of menadione sodium bisulfite (10mg/kg b.wt) for two consecutive days, the owner complaints were a colicky pain, signs of discomfort, anorexia, poor performance, rough coat, red urine and oliguria.
Animal Grouping
A total of 17 draft horses were used in the present study; 10 apparently healthy animals (4 males and 6 females)aged 4-15 years old belonged to private farms were used as a control group. The next group includes 7 draft horses (3 males and 4 females), of which four horses aged 4-6 years old from one locality (the field cases mentioned above) were admitted to the Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Zagazig University, Egypt. The other three draft horses aged 13 and 15 years old (experimental cases). Experimental cases were administered menadione sodium bisulfite simulator to the field cases. the four field cases and the three experimental cases were actually exposed to oral administration of vitamin k3, (vitamin K3-exposed group).
Ethical Approval
Animal approaching, dosing, and sampling were applied and approved ethically using guidelines for the ethical use of Animals in Teaching Research Committee of Zagazig University.
Clinical Examination
Thorough clinical examination was performed; the history of the clinical cases was taken regarding age, location, feeding, previous illness, medications and the observed signs. Preliminary general examination to all cases was done, and vital signs were monitored, rectal examination to the left kidney was also performed according to Kelly (1984).
Abdominal Ultrasonography
Abdominal ultrasonography (US) in horses was applied by an ultrasound scanner with the convex probe 2.5-3.5 MHZ and transrectal ultrasonography with a linear array probe 6-8 MHZ was performed. Ultrasonographic coupling gel (SGMOSCAN, SGMO Chemical Industry, Egypt) was used. Ultrasonography of kidneys was done from the left and right body walls at the 14th -17th intercostal space (ICS) for right kidney and the 17th ICS- para-lumbar fossa for left kidney using a 3.5 MHZ transducer. Scanning was carried out in both sagittal and transverse plans (Hoffman et al., 1995).
Urinalysis
Urine samples were collected from horses of both groups either spontaneously or by a sterile urethral catheter. The collected urine sample from each animal was divided into two portions; the 1st portion was used for complete urinalysis (physical, chemical and microscopical) meanwhile, the 2nd portion was centrifuged using K243R refrigerated centrifuge, UK (3000 rpm, 10 min, and 4oC), then sediment was discarded and clear supernatant was collected and frozen at-20oC for clinico-biochemical analysis such as urinary protein using ProteoSpin™ urine protein concentration micro kit with CAT. NO. 17400 (Martin 2011).Urinary glucose was measured using enzymatic colorimetric (GOD-POD) BioMed-Glucose L.S kits with REF. NO.GLU109480according to the method of Trinder (1969). Urinary creatinine (Cr) was determined using BioMed- Creatinine kits with REF. NO.CRE 106100 according to the method of Henry et al. (1974). Urinary gamma-glutamyl transferase (GGT) was detected by Kinetic kits of EGY- CHEM for lab technology, Egypt with REF. NO.GGT 124100 according to the method of Szasz et al. (1994). While the colorimetric kits with CAT. NO. MDA 25 29of Bio diagnostic Co, Egypt was used to determine the content of urinary malondialdehyde (MDA) according to the method of Satoh (1978). Urinary nitric oxide (NO) was determined by the method of Montgomery and Dymock (1961) using the colorimetric kits of Biodiagnostic Co, Egypt.
Clinicopathological Tests
Two blood samples were collected from a jugular vein of all examined horses, the first sample (5 ml) placed on EDTA-tube for hematological analysis (hemogram and leukogram) using full version automatic cell counter (Sysmex KX-21N, Japan). According to the method of Grindem (2011). The second blood sample (5 ml) was collected on clean, dry, and sterile centrifuge tube to separate serum for determination of serum Cr (Henry et al., 1974), urea (Vassault et al., 1986), uric acid (Fossati et al., 1980) and albumin (Doumas and Biggs, 1976). Moreover, serum sodium (Na+) was measured colorimetrically using an endpoint BioMed-Sodiumk its with REF. NO. SOD100100 according to the method of Maruna (1958), serum magnesium (Mg++) by colorimetric, endpoint BioMed-Magnesium (Single Reagent) kits with REF. NO. MG122050 (Bohuon, 1957). Furthermore, creatinine clearance was calculated by dividing the volumeurine × creatinineurine on creatinineserum ×1,440 (Rumpler et al., 2013). Serum iNOS activity was measured using the technique previously applied by Bredt et al. (1991).
Postmortem Finding and Histological Examination
Postmortem findings were recorded, and small kidney fragments were fixed in 10% neutral buffered formalin. Thereafter, routine paraffin wax blocks were prepared for each specimen, and then 5µm thick tissue sections were obtained in order to stain with hematoxylin and eosin (HE) for light microscopical examination (Suvarna et al., 2013).
Statistical Analysis
Data were statistically analyzed by a statistical software program (SPSS, version 16) using independent t-test. Mean ±SE for each parameter were estimated. Results were considered statistically significant at P < 0.05 (Duncan, 1995).
Results
Clinical Signs
Results of clinical examination were summarized in Table 1. In both field and experimental cases within 4-24 hs of the 2nd dose of vitamin K3, horses develop non-specific signs of dullness, became anorexic, depressed, weak with poor performance. After that, a characteristic renal colic was observed in which the horse stand arched back and sometimes look at their flank, horses lie down and get up frequently, paddling by their legs on the ground and stranguria may be followed. Vital signs monitoring in all cases revealed a significant increase in heart, respiratory rates, and rectal temperature in the vitamin K3-exposed group in comparison with control ones. Rectal palpation revealed enlargement in left kidney with pain on palpation. Right kidney could not be reached in any one.
Renal Ultrasound Report
Ultrasonically, Kidneys of control horses were normal in size and showing normal medullary and cortical echogenic pattern. The left kidney has a more elongated shape (bean-shaped form). While, the right kidney has a curvilinear triangle shape (heart shape). In vitamin K3-exposed group, enlargement of the kidneys was the common findings with loss of differentiation between cortex and medulla in 40% of cases (Figure 2A).While increase the echogenicity of the renal cortex was imaged in 60% of diseased cases, making the corticomedullary junction more prominent (Figure 2B).
Urinalysis Results
As shown in (Table 2),urine samples from vitamin K3-exposed group physically were dark red with persistent red foam formation (Figure 3A). While chemically showed severe proteinuria (+++), hematuria (+++) with mild glucosuria (+) and pyria (+) compared with the control group which showed normal turbid urine. Furthermore, microscopical examination of urine in vitamin K3-exposed group revealed extensive crystalluria, it characterized by the presence of much slightly brown calcium carbonate crystals with different shapes (hexagonal, spherical and dumble) (Figure 3B). A huge number of red blood cells (≤ 100 cells /HPF) were also detected (Figure 3C).
Table 1: Clinical findings in healthy and vitamin K3 -exposed group
| Variable | Control group (n=10) | Vitamin K3-exposed group (n=7) | |||
| Appearance | Alert | Dull and depressed (7 of 7) | |||
| Lethargy | Absent | Little lethargic (2 of 7) to great lethargic (5 of 7) | |||
| Performance | Good | Poor (7 of 7) | |||
| Appetite | Good | Inappetence (3 of 7), Anorexia (4 of 7) | |||
| Body weight loss | Absent | Present (5 of 7) | |||
| Mucous membrane color | Rosy red | Pale (5 of 7)& Rosy red (2 of 7) | |||
| Vital signs | Pulse rate | 29.2±1.65 | 34.40±1.88** | ||
| Resp. rate | 12.2±0.66 | 20.8±1.01** | |||
| Body temp. | 37.56±0.05 | 38.8±0.61** | |||
| Loss of luster of hair | Absent |
Present (4 of 7) |
|||
| Colic | Absent | Mild colic (3 of 7), Moderate colic (4 of 7). | |||
| Urination | Stranguria | Absent | Present (5 of 7) | ||
| Urine | Volume/24hrs | Normal | Oliguria (6 of 7) | ||
| Color | Straw yellow | red | |||
| Defecation | Normal | Normal (6 of 7), soft (1 of 7) | |||
| L. kidney rectally | Normal size |
Increased size with pain (7 of 7) |
|||
Table 2: Physical and chemical urinalysis of vitamin K3-exposed group compared to control group
| Control group | Vitamin K3>-exposed group | ||
| Physical urinalysis: | |||
| Volume(ml/kg b. wt/day) | 8-10(n=10) |
3-5(n = 7) |
|
| Color | Ochre (n= 10) |
Ochre (n = 0), Red (n=7) |
|
| Odor | Aromatic (n=10) |
Aromatic (n=7) |
|
| Aspect | Turbid (n =10) |
Turbid and dark red (n=7) |
|
| Foam | Abscent (n = 10) |
Abscent (n = 0), Red persistent (n = 7) |
|
| Chemical urinalysis: | |||
| pH | 7.6-9.0 |
7.6-9.0 |
|
| Specific gravity | 1.020-1.040 |
1.030-1.045 |
|
| Glucose | -ve |
+ (50 mg/dl) |
|
| Protein | -ve |
+++ (500 mg/dl) |
|
| Leukocytes | -ve |
+ (25 Leuko/µl) |
|
| Blood | -ve |
+++ (250 Ery/µl) |
|
Hematological Findings
The vitamin K3-exposed group showed non-significant changes in red blood cells (RBCs), lymphocytes, monocyt-
es, Hb concentration, packed cell volume (PCV), mean corpuscular volume (MCV) and mean corpuscular hemog lobin concentration (MCHC). On the other hand, the same group recorded a significant (P<0.05) elevation in the counts of white blood cells (162.16%), neutrophils (88.49%) and eosinophils (105.71%) compared to control (Table 3).
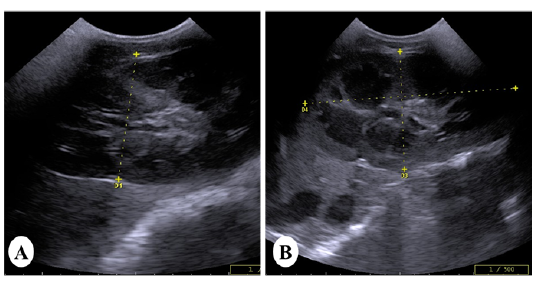
Figure 2: Ultrasound images of the kidney of vitamin K3-exposed horses showing (A); enlargement of the right kidney with loss of differentiation between cortex and medulla whereas, the left kidney showing increasein the echogenicity of the renal cortex making the corticomedullary junction more prominent (B)
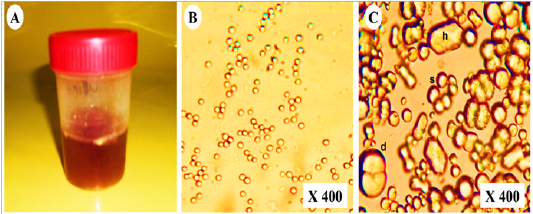
Figure 3: Representative photo showing (A); dark red urine contained (B); non- organized dumble (d), spherical-radiated(s) and hexagonal(h) shaped calcium carbonate crystals with (C); organized erythrocytes in the urine of vitamin K3-exposed horses
Table 3: Erythrogram and leukogram of vitamin K3-exposed group compared to control group (Mean values ±SD)
| Control group | Vitamin K3-exposed group | ||||
| Erythrogram: | |||||
|
RBCs(×106/µl) |
9.44a±0.19 |
9.90a±0.17 |
|||
| Hb(g/dl) |
14.60a±0.16 |
14.80a±0.14 |
|||
| PCV(%) |
47.60a±0.92 |
49.00a±1.89 |
|||
| MCV(fl) |
50.42a±1.00 |
49.49a±1.26 |
|||
| MCHC(%) |
30.67a±0.28 |
30.20a±1.27 |
|||
| Leukogram (×103/µl): | |||||
| WBCs |
5.80b±0.46 |
9.40a±0.62 |
|||
| Lymphocytes |
2.24a±0.25 |
2.64a±0.38 |
|||
| Neutrophils |
3.13b±0.35 |
5.90a±0.33 |
|||
| Eosinophils |
0.35b±0.05 |
0.72a±0.06 |
|||
| Monocytes |
0.13a±0.02 |
0.14a±0.04 |
|||
| Baophils | 0.00±0.00 | 0.00±0.00 | |||
Variables with different superscript in the same raw are significantly different at P < 0.05. Red blood cells (RBCs), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC)
Urine and Serum Biochemical Changes
Table 4, showed a significant (P<0.05) increase in urinary MDA (221.37%), GGT (175.32%), protein (233.50%), glucose (59.30%), and creatinine (231.35%) as well as serum albumin (50.73%), sodium (412.52%), urea (139.81%), uric acid (125.08%) and creatinine (574.39%)in the vitamin K3-exposed group. On the contrary, there was a significant (P<0.05) decrease in serum magnesium (-54.21%). Furthermore, Figure 4, showed a significant (P < 0.05) decrease in creatinine clearance with significant (P < 0.05) elevation in serum nitric oxide synthase activity and urinary nitric oxide (Ur.NO) level in vitamin K3-exposed group in comparison with control group.
Gross and Histopathological Observations
In post-mortem examination, kidneys of vitamin K3-exposed group were enlarged, congested and less firm on palpation than normal (Figure 5A,B). Microscopically, they showed medullary lymphocytic aggregations (Figure 6A), cortical lymphocytic aggregations with marked necrosis of the surrounding renal tubules (Figure 6B), globular eosinophilic proteinisuos casts in the majority of the intact renal tubules (Figure 6C). Proliferative glomerulonephritis with the presence of protein casts inside the renal tubules was also detected (Figure 6D). Tubular casts of polymorph nuclear cells and cystic dilation of the surrounding tubules were detected (Figure 6E). Periglomerular fibrosis with marked proliferation of the mesangial cells and globular protein casts in the renal tubules were also observed (Figure 6D).
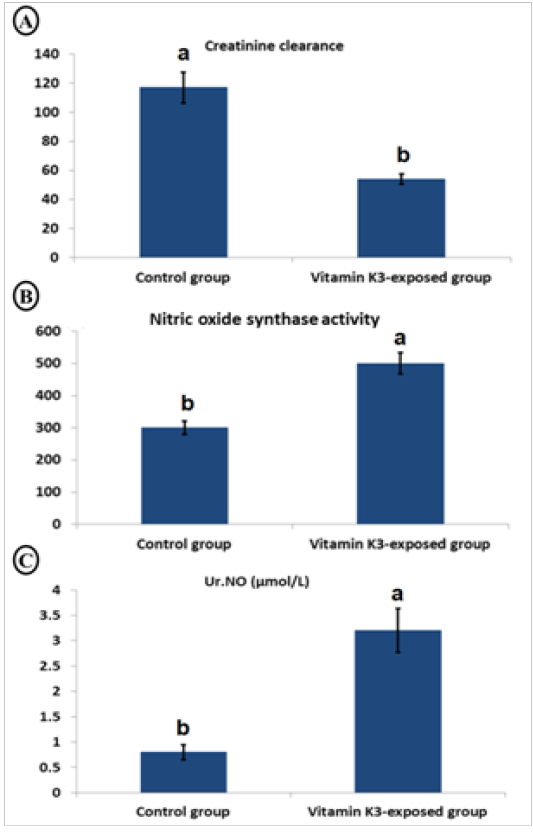
Figure 4: Char bars showing changes in creatinine clearance (A), serum nitric oxide synthase activity (B) and urinary nitric oxide (Ur.NO) (C) in vitamin K3-exposed horses comparing with the control horses
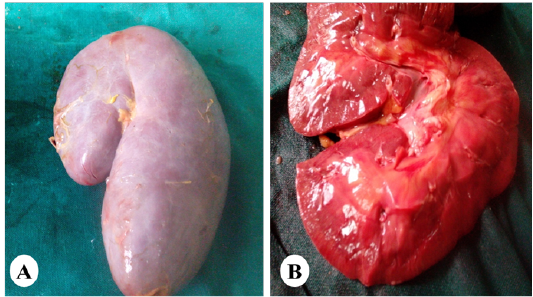
Figure 5: Representative photo showing enlargement of the kidney (A) with cortical and medullary congestion (B) of horses exposed orally to vitamin K3.
Table 4: Selective serum and urinary constituents of vitamin K3-exposed group compared to control group ( Mean values ±SD)
| Control group | Vitamin K3-exposed group | |
| Urinary: | ||
| MDA(µmol/L) | 2.62b±0.14 |
8.42a±0.35 |
γGT(U/L) |
15.40b±0.14 |
42.40 a±1.02 |
| Protein(mg/dl) | 154.20b±1.35 |
514.26 a±7.62 |
| Glucose (mg/dl) | 34.40 b±1.63 |
54.40 a±1.36 |
| Creatinine (mg/dl) | 15.50 b±0.17 |
51.36 a±1.17 |
| Serum: | ||
| Albumin (g/dl) | 5.46b±0.17 |
8.23a±0.29 |
Na+(mEq/l) |
9.26 a±0.20 |
4.46 b±0.35 |
Mg++(mg/dl) |
1.90 a±0.03 |
5.87 b±0.02 |
| Urea(mg/dl) | 22.10 b±0.71 |
53.00 a±1.14 |
| Uric acid(mg/dl) | 5.98 b±0.13 |
13.46 a±0.45 |
| Creatinine (mg/dl) | 0.82 b±0.01 |
5.53 a±0.52 |
Variables with different superscript in the same raw are significantly different at P≤ 0.05
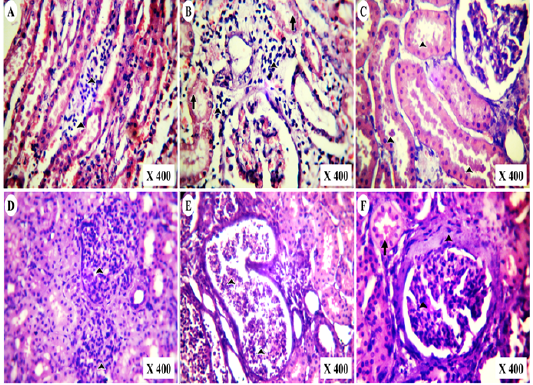
Figure 6: Photomicrographs of HE-stained kidney section of vitamin K3-exposed group showing (A); medullary lymphocytic aggregations (arrowheads),(B); cortical lymphocytic aggregations (arrowheads) with marked tubular necrosis and (C); globular eosinophilic proteinisuos casts (arrowheads) Also, the experimental cases showing (D); proliferative glomerulonephritis (arrowheads) with protein casts inside the renal tubules, (E); tubular casts of polymorph nuclear cells (arrowheads) and tubular cystic dilation besides (F); periglomerular fibrosis with mesangial cells proliferation (Arrowheads) and globular protein casts (arrow)
Discussion
Nowadays, acute renal injury (ARI) in human and animals may be suggested to be associated with an inflammatory response which resulted in cellular oxidative and nitrosative damage (El-Ashker et al., 2014). In equine, vitamin K3 nephrotoxicosis is an important cause for renal failure (Maxie et al., 1992). Menadione is a synthetic vitamin K3 and found to be nephrotoxic in equine at a dosage of 1-2.5 g (Green and Green, 1986).The pathogenesis of vitamin K3-induced nephrosis is not fully understood, but it may be attributed to direct acute tubular damage and/or indirect hemoglobinuric nephrosis (Rebhun et al., 1984).The prognosis is usually poor because of interstitial renal fibrosis caused by vitamin K3 toxicity in horses (Maxie et al., 1992). Advanced search about the possible mechanism for toxicity was necessary and required. Thus, the present study was established in a trail to find the nephrotoxic pathogenesis of menadione with a dose of 10mg/kg b.wt in horses.
It is suggested that horses orally administered vitamin K3 showed signs of dullness, depression, weakness, lethargy, and anorexia which may go unnoticed. Similar clinical findings were reported by Rebhun et al. (1984), following intravenous or intramuscular injection of the manufacture recommended dose of vitamin K3 (2.2 to11mg/ kg BW).Maxie et al. (1992) recorded that menadione induced depression, colic, muscle stiffness, and azotemia when adult horses were injected intravenously menadione sodium bisulfite at 200 mg/animal in order to control exercise-induced pulmonary hemorrhage. These signs could be attributed to the accumulation of wastes in the body and marked azotemia, as the severity of clinical findings were varied between the diseased horses according to the degree of azotemia. Vital signs alterations, false colic and the detected abnormality in the kidney rectally could be attributed to acute nephropathy caused by vitamin K3 administration.
Acute nephropathy in horses probably results from oxidative and nitrosative renal damage induced by menadione by accelerating the reactive oxygen species (ROS)/ reactive nitrogen species (RNS) generation in the kidney. Oxidative stress is characterized by increased ROS and/or RNS which were indicated in both human and animal models with kidney injury (Ratliff et al., 2016). In the kidney, nitric oxide (NO) plays a great role in the regulation of physiological glomerular functions (Prabhakar, 2001). Many studies indicated that NO activates and regulate the cellular ATP generation, mitochondrial respiration and membrane transport activity in normal kidney tissue (Guilivi et al., 1998). NO is synthesized by the action of three catalyzing enzymes [endothelial (eNOS), neuronal (nNOS), and inducible nitric oxide synthase (iNOS)] which produce the largest amount of NO (Sugimoto et al., 1999). NO also plays a vital role in the endothelium during oxidative stress caused glomerulonephritis (GN) as it may be involved in pro-inflammatory chemokines, peroxynitrite formation, and signaling pathways besides its direct glomerular effects which promote the albumin permeability in GN(Li et al., 2001). Gomez-Guerrero et al.(2002) reported that iNOS derived NO may promote inflammatory injury in GN). Consequently, infiltrating leukocytes (including neutrophils and macrophages) invade the glomerulus and perivascular milieu of kidney contributes to the renal tissue prodamage by ROS (Rosen et al., 2002). These leukocytes release large amounts of pro inflammatory cytokines such as tumor necrosis factor-alpha (TNFα), interleukin-1beta (IL-1β) and, and undergo a respiratory burst, which includes hypochlorous acid, hydroxyl radical-like species, hydrogen peroxide, nitric oxide (via inducible nitric oxide synthase [iNOS]), and nitrogen dioxide (Yang, 2011).
Another probable reason for acute nephropathy of menadione is serum albumin which is the major protein in plasma constituents and aids in the contributing or preventing oxidative stress in the proximal renal tubules. It found to have antioxidant activity at lower concentrations but at higher levels, it induces ROS liberation and promotes bad effects such as glomeruli injury or glomerular filtration barrier by stimulation of the protein kinase C (PKC) activity, followed by activation of (NOX4) NADPH oxidase and generation of superoxide. High levels of albumin also enhance its binding to fatty acids, which effectively impairs SOD activity in PTCs while promoting mitochondrial ROS production (Shalamanova et al., 2007). The increase in ROS in the cytosol of PTCs activates STAT, NFkB, and AP-1 intracellular signaling cascades, leading to expression of pro inflammatory genes and promotion of interstitial fibrosis in the kidney (Terryn and Devuys, 2011).
All the above may explain our findings which revealed that vitamin K3-exposed group showed a significant increase in the urinary levels of malondialdehyde (lipid peroxidation marker), nitric oxide and iNOS activity. The observed renal damage caused by menadione resulted in azotemia, proteinuria, hematuria, glucosuria and elevated serum creatinine, gamma-glutamyl transferase enzyme activity which is a glycoprotein observed in cells with secretory function, such as the proximal renal tubular cells. It has pro-oxidant activity by modulation of hydroxyl radicals’ production and increasing the membrane lipid peroxidation (Kwiatkowska et al., 2014). There is a great relationship between body electrolyte balance and renal function. Renal failure is often complicated by elevations in magnesium and decreases in sodium (Miller et al., 2009). The obtained biochemical results were confirmed by the observed histopathological alterations and ultrasonographic findings.
Ultrasonography is an important diagnostic imaging technique in the investigation of horse abdominal disease, ultrasonography of the kidney is important to diagnose renal disease in horse especially when unilateral involvement is expected, as the animal may have normal serum biochemistry (Alexandra et al., 2011; Habershon-Butcher et al., 2014). In the present study, enlarged kidneys with or without increasing the echogenicity of the cortex usually indicate acute renal injury. Overall, changes in echogenicity of the kidney alone are not enough for distinguishing the causes of AKI. As the disease may or may not associated with an increase in the echogenicity of the cortex, other points are helpful in the diagnosis as, enlargement of the kidney and clinical findings, at these points certain disease may be more likely than others.
Regarding the hemogram, vitamin K3-exposed group showed non- significant changes in erythrogram with leukocytosis, neutrophilia and eosinophilia compared to the control group. Ischemic, nephrotoxic, and endotoxemia-induced ARI are associated with an increase in infiltrating neutrophils in the kidney (Cunningham et al., 2004). There is evidence that neutrophils mediate tubular injury in AKI and play a key role in the development of acute renal failure (Bolisetty and Agarwal, 2009).
Conclusion
The results of the present study indicated that vitamin K3 in horses induces oxidative and nitrosative renal damage resulting in ARI characterized by azotemia, proteinuria, and hematuria and decreased creatinine clearance. In spite the importance of vitamin K3 in the horse, vitamin K3 must not be administered to the horse by any route.
AcknowledgementS
Authors were greatly thankful to Dr/ Haitham Ali, Lecturer of Pathology, Faculty of Veterinary Medicine, Zagazig University for his aid in reading the histopathological alterations.
Conflict of interest
There are no conflicts of interest and no funds.
Authors Contribution
All authors has planned and conducted the study, WAM analyzed the data. WAM and YHB wrote the manuscript with input from NEA. All authors discussed the results and contributed to the final version of the manuscript.
Informed consent
There are no studies with human subjects.
References