Advances in Animal and Veterinary Sciences
Research Article
Assessment of Suturing Technique, Gelatin Sponge and Aluminum Chloride in Controlling Hepatic Bleeding and Abdominal Adhesions in Dogs
Alaa Ghazy*1, Ayman Atiba1, Walied Kotb2
1Department of Surgery, Anesthesiology and Radiology; 2Department of Pathology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, El Geish Street, 33516, Egypt.
Abstract | Control of bleeding in liver surgery is a challenging procedure. The hemostatic time, gross lesions, and histopathological effects of topical hemostatic agents (Gelatin Sponge and Aluminum Chloride) were investigated in comparison with the standard suturing technique in an induced liver wound in dog. Eighteen dogs of both sexes were divided into 3 groups; Group (A) suture group, Group (B) gelatin sponge and Group (C) Aluminum Chloride (3 male and 3 female per each group). Results: Hemostasis time was significantly shorter in dogs treated by topical hemostatic agents in Group (B) and Group (C) in comparison to Group (A) (P < 0.001). Hemostasis times in Group (C) were significantly less than those of Group (A) and Group (B) (P < 0.001). Grossly, peritoneal adhesion was observed extensive in Group (A), moderate in Group (B), and minimal in Group (C). Histopathologically, group (A) showed marked necrobiotic changes within the hepatic tissue associated with intra- and extra-lobular fibrosis. Group (B) demonstrated an over granulation tissue formation accompanied with persistent gelatin sponge spicules and obvious inflammatory reaction. While in Group (C), most dogs revealed an advanced degree of healing signs with minimal hepatic tissue injury. Aluminum Chloride compared to Gelatin Sponge and suturing the liver, offers an effective local hemostatic action to control bleeding in a liver tissue.
Keywords | Gelatin sponge, Aluminum chloride, Liver, Bleeding, Dog
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 08, 2016; Accepted | January 18, 2017; Published | February 13, 2017
*Correspondence | Alaa Ghazy, Kafrelsheikh University, Egypt; Email: alaa.abdelsalam@vetkfs.edu.eg
Citation | Ghazy A, Atiba A, Kotb W (2017). Assessment of suturing technique, gelatin sponge and aluminum chloride in controlling hepatic bleeding and abdominal adhesions in dogs. Adv. Anim. Vet. Sci. 5(2): 56-61.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.2.56.61
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Ghazy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The rich hepatic vascular network makes the control of bleeding liver is a challenging procedure. The liver needs to be cut in many operations (Makuuchi et al., 2002; Jarnagin et al., 2004). Inadequate hemostasis is the main cause of death in hepatic resection (Romano, 2013; Barbas, 2013; Wei, 2003; Rahbari, 2011) and increases the operative time, hospital stay, and recovery (Shander, 2007). Postoperative intestinal adhesions caused mainly by blood that remain in the abdominal cavity after surgery (Ryan et al., 1971). This has motivated a lot of studies to introduce new techniques in controlling hemorrhage in liver tissue (Goodman and Gilman, 1980; Berrevoet, 2007; Jackson, 2001; Berrevoet and de Hemptinne, 2007).
Suturing the lacerated liver with deep stitches is the standard method used to control bleeding (Carrillo and Richardson, 2001). Using sutures to control bleeding in liver can cause parenchymal injuries in normal liver. On the other hand, the liver parenchymal is not a favourable tissue for suturing; and with an inexperienced surgeon, the sutures will increase the incidence of liver parenchyma rupture (Nouri and Sharif, 2015).
Most of the topical hemostatic agents used to control bleeding from the liver tissue require normal homeostatic function to providing a matrix for coagulation (Emilia et al., 2011). Gelatin Sponge is a sterile compressed sponge that has hemostatic properties. When applied directly to bleeding surfaces, it forms an artificial clot and producing a mechanical matrix that facilitates clotting (Rarig, 1963; Jenkins and Janda, 1946; Jenkins et al., 1946). Barnes (1963) studied the uses gelatin sponge in gynecologic surgery. Gelatin sponge provides a protective cover and structural support for the reparative process in canine liver resections (Jenkins and Janda, 1946; Guralnick and Berg, 1948). The damaged platelets release thromboplastin, which give the gelatin sponge its clotting effect. Thromboplastin interacts with prothrombin and calcium to produce thrombin, initiating the clotting reaction (Jenkins et al., 1946). The spongy physical properties of gelatin sponge hasten clot formation (Guralnick and Berg, 1948). Several researchers have reported that gelatin sponge becomes liquefied within a week or less and is completely absorbed in four to six weeks, without inducing excessive scar formation (Goodman and Gilman, 1980; Jenkins et al., 1946; Jenkins and Janda, 1946; Treves, 1952; Rarig, 1963). The gelatin remnants can induce a foreign body reaction, chronic inflammation, or infection leading to formation of granuloma and interfere with the wound healing process (Samudrala, 2008).
Aluminum Chloride (AlCl3) is a chemical agent with acidic property frequently used as a hemostatic agent to control the local bleeding in dental surgeries (Nouri et al., 2014) and liver lacerations (Nouri and Sharif, 2014). Aluminum Chloride, unlike the well-known hemostatic agents, exerts its hemostatic effect through a chemical reaction with blood and does not need normal body hemostatic system to exert its effect, so it is a very efficient hemostatic agent (Nouri and Sharif, 2014). Moreover, the acidic property of Aluminum Chloride should be considered too. It coagulates the blood proteins rapidly and makes a barrier and closes the opening of small blood vessels (Porte et al., 1989). This barrier blocks entrance of aluminum chloride into the vessels and hence, prevents its potential systemic side effects (Nouri et al., 2013). Many studies indicated the efficacy of aluminum chloride in decreasing the hemostatic time during surgery (Heaton, 2005; Nouri et al., 2014).
The aim of the current study was to determine the hemostatic and pathological effect of Gelatin Sponge and Aluminum Chloride in controlling bleeding from liver in a canine animal model, to compare them with the standard method (suturing technique), and to assess the gross changes in the abdominal cavity after surgery.
Materials and methods
Animals
This study was performed at the Faculty of Veterinary Medicine, Kafrelsheikh University. In this study, 9 males and 9 females dogs, weighing 15-20 kg were used. All animals were tested before surgery for prothrombin time (PT) and activated partial thromboplastin time (PTT), and liver function tests (AST, ALT) to make sure that they are normal. Animal handling and all the experiments were approved by the local research council of Kafrelsheikh University, Kafrelsheikh, Egypt.
Dogs were divided into 3 groups, each containing 3 males and 3 female dogs, Group (A) suture group, Group (B) Gelatin Sponge group, and Group (C) Aluminum Chloride group.
Surgery
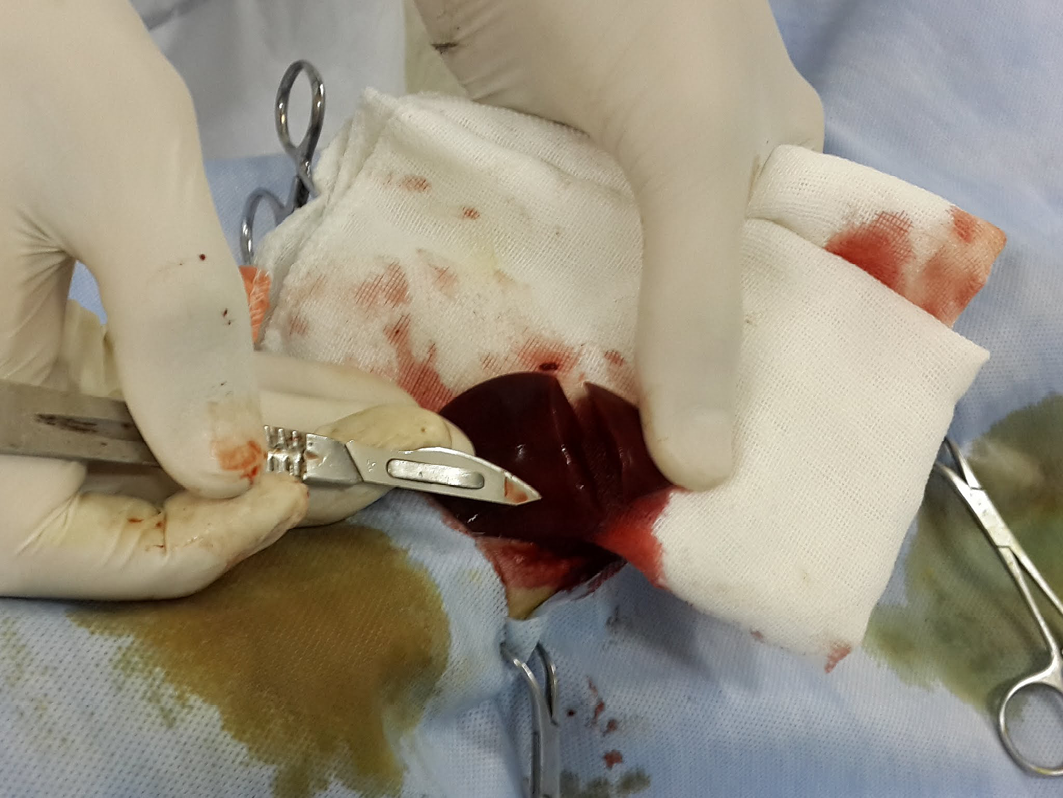
The dogs were initially sedated by intravenous injection of 1mg/kg xylazine HCl (Xylaject 2%, ADWIA, Egypt), and then anesthetized by ketamine HCl 10mg/kg (Ketamax-50, Troikaa Pharmaceuticals Ltd., India), and surgically prepared. Thereafter, a ventral midline laparotomy incision was made and the caudate lobe of the liver was extracted from the abdominal cavity. Next, a 3-4 cm incision with 1/2 cm depth was made on the caudate lobe of the liver by a scalpel and the depth was determined by a mark made on the scalpel 1/2 cm from the tip, and the length was measured with a ruler on the liver as described in (Figure 1).
The depth was determined by a mark made on the scalpel 1/2 cm from the tip (the arrow), and the length was measured with a ruler on the liver 3-4 cm
In Group (A), the liver was closed with simple sutures using Silk 3-0 to control bleeding. All sutures were performed by the same surgeon. In group (B), a piece of Gelatin Sponge was cut to the desired size, applied dry to the cut surface of liver, and held in place with moderate pressure until complete hemostasis. In Group (C), Aluminum Chloride 25% was dropped on the cut surface of liver until complete hemostasis.
After complete hemostasis, the hemostasis time (time required for complete drying and no blood oozing from the cut surface of liver) was calculated in every single animal. The abdominal wall was closed in a routine manner, and each animal received a single I.M injection of 500 mg of cephtriaxone (Mesporine, Sigma, Egypt) daily for 3 successive days to prevent infection.
All animals were euthanized at the 15th postoperative day using IV injection of a high dose of thiopental sodium (Thiopental sodium 1g, Epico, Egypt). The abdominal cavity was explored to detect adhesions or collections of clotted blood. A liver section from caudate lobe was obtained from every single animal.
Histopathology
The liver sections were fixed in 10% neutral buffered formalin and trimmed into 3-mm-thick slices. After the tissue was dehydrated in series of alcohol, cleared in xylene, and embedded in paraffin, it was cut into 4 µm sections and was stained by haematoxylin/eosin (HE). The histological evaluation of the different sections was assessed blindly. Quantitative assessment of areas of hemorrhage, inflammation and fibrosis were done using Image-J analysis software (National Institutes of Health, MD, USA), and the data was expressed as mean area (mm2/cm2 ± SD).
Statistical Analysis
Data were entered into SPSS software and followed by pairwise multiple comparison test Tukey-Kramer. All results were considered significant for comparative values (p < 0.05).
Results
Hemostatic Results
The study showed that, compared to group (A), the hemostasis time was significantly shorter in Group (B) and (C) at which a local hemostatic agents were used (P < 0.001).
Hemostasis times of the three groups are shown in Table 1. Complete hemostasis occurred in three groups. Hemostasis times in Group (C) were significantly less than those of Group (A) and group (B) (P < 0.001). Hemostasis times in Group (B) were significantly less than those of Group (A).
Table 1: Hemostasis Times (in seconds) of Suturing Technique, gelatin spong, and Aluminum chloride 25% in Liver Parenchyma
|
Group |
Hemostatic Time |
|
(A) Suture |
82.4 ± 4.8 |
|
(B) Gelatin Sponge |
28.5 ± 5.3 |
|
(C) Aluminum chloride 25% |
15.2 ± 4.1 |
|
P value |
< 0.001 |
A data are presented as mean ± SD
Gross/PM Results
The injured part of the liver adhered extensively with the peritoneum in Group (A), moderately in Group (B), and very lightly in Group (C). (Figure 2-A, B, and C, respectively).
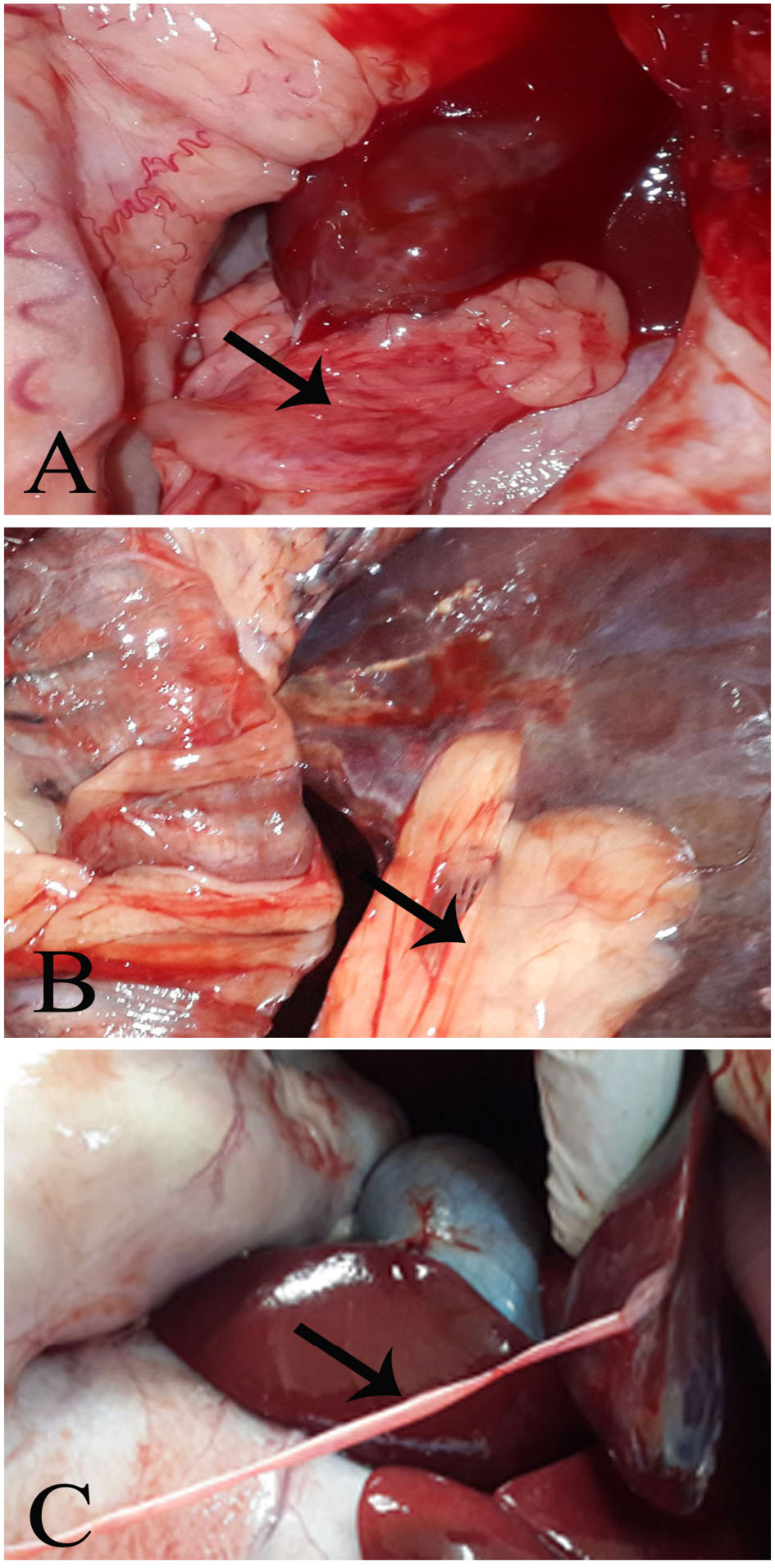
Figure 2: Post mortum examination
Group A, showing a massive adhesion. Group B, showing a moderate hepatoperitoneal adhesion. Group C, showing thin layer of adhesion tag (fibrin tag).
Histopathological Findings
Quantitative assessment of the hepatic necrosis, inflammation and fibrosis was illustrated in Table 2. The Group (B) and (C) showed significant decrease of the necrosis, hemorrhage and inflammation areas in comparison with Group (A) and (B) (P < 0.001). While animals of Group (C) revealed marked significant decrease of necrosis, hemorrhage and fibrosis in comparison with Group (B) (P < 0.001).
Table 2: Image analysis of necrosis, inflammation, and fibrosis within different treated
|
Groups |
Necrosis |
Inflammation |
Fibrosis |
|
|
(A) |
Suture |
18.45±3.12 |
15.45±4.56 |
20.74±6.28 |
|
(B) |
Gelatin Sponge |
12.16±1.94a |
11.81±2.19a |
13.10±3.82a |
|
(C) |
Alum. chloride 25% |
6.30±1.07a,b |
4.62±2.18a ,b |
5.93±1.74a ,b |
A data are presented as mean ± SD
Histologically, the liver sections from animal of Group (A) showed presence of non-absorbable suture filaments within the hepatic tissue associated with hepatic degeneration mostly of fat type, multifocal areas of necrosis, hemorrhages and extensive fibrosis which extended deep within the hepatic parenchyma (Figure 3–A, B).
Liver sections from animals treated with Gelatin Sponge in Group (B) revealed apparent residual gelatin sponge spicules associated with intense inflammatory reaction represented with congestion of the blood capillaries, fibrin deposition and marked inflammatory cells infiltration mostly lymphoplasmacystic and macrophage cells. Also, over granulation tissue formation accompanied with marked angiogenesis (Figure 3C). The most of hepatic tissues were within normal limits unless the adjacent areas to the wound demonstrated vacuolation of their hepatocytes (Figure 3–D)..
Liver sections from animals treated with aluminum chloride in Group (C) showed small-sized compact protective scab with minimal degree of vascular congestion, granulation tissue formation and leukocytic infiltration associated with normal hepatic tissues (Figure 3E, F).
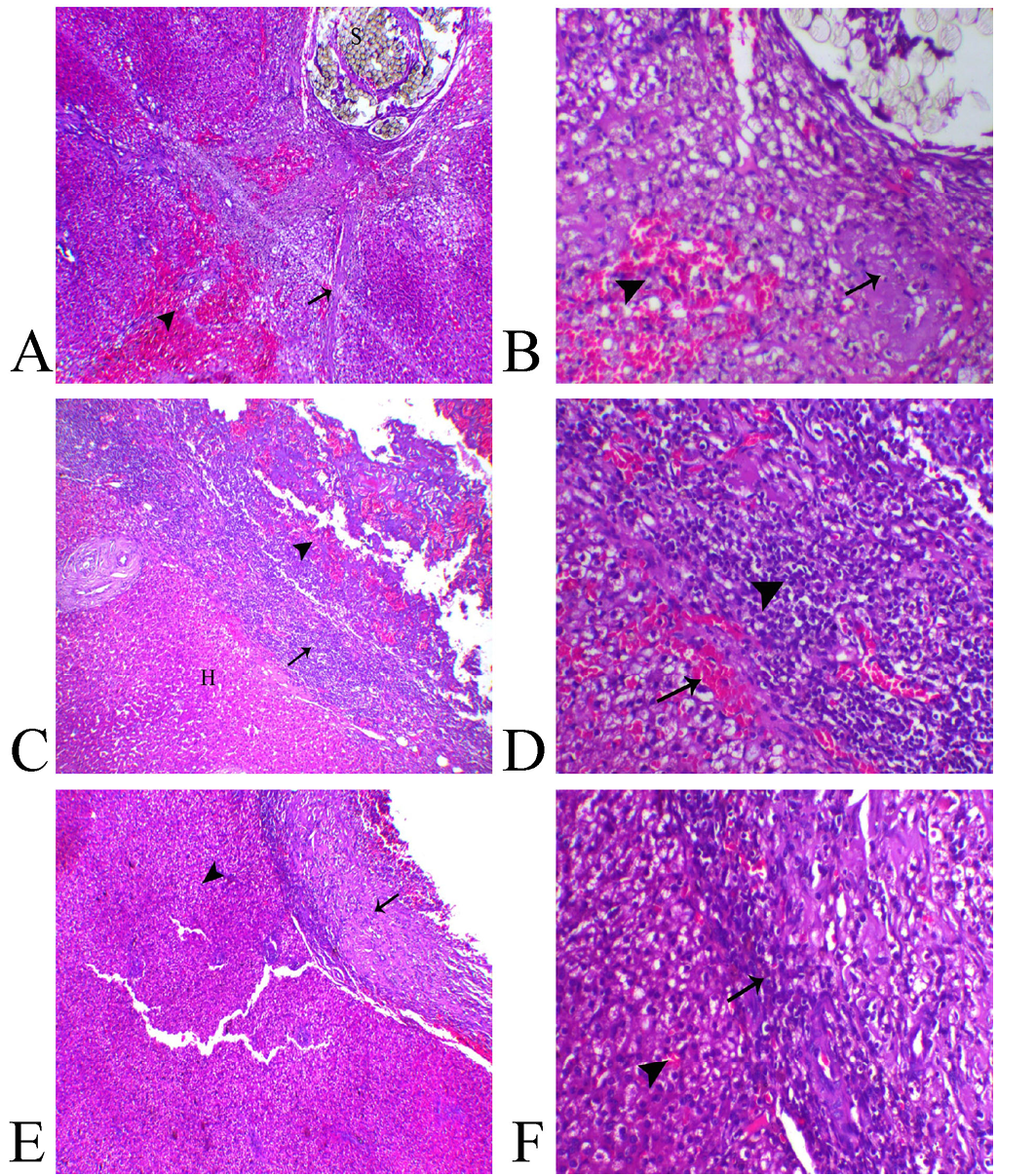
Figure 3: Photomicrograph of liver sections A, B from (Group A), C, D from (Group B) and E, F from (Group C).
A) Liver of Group A showing suture filaments (S) in association with marked diffuse fibrosis (arrow) and hemorrhage ((arrowhead), H&E, X40, B) higher magnification revealing focal hepatic necrosis (arrow) and hemorrhage ((arrowhead), H&E, X200. C) Group B showing normal hepatic tissue (H), over granulation area formation (arrowhead) separated with inflammatory zone ((arrow), H&E, X40, D) higher magnification showed marked inflammatory reaction represented with vascular congestion (arrow) and intense leukocytic infiltration ((arrowhead) H&E, X200. E) Group C showing normal hepatic parenchyma (arrowhead) and minimal areas of granulation and inflammation (arrow) H&E, X40, F) higher magnification showing mild leukocytic infiltration (arrow) and normal hepatic tissue ((arrowhead) H&E, X200).
Discussion
The present study aimed to compare the hemostatic effect of gelatin sponge, aluminum chloride and the slandered simple suturing in controlling bleeding in induced cut surface of liver in dogs. The study showed that compared to suturing in Group (A), the hemostasis time was significantly shorter in Group (B) and (C) at which local hemostatic agents were used. According to Kim and Rethnam, a hemostatic agent that stops bleeding in the shortest possible time, is the good one (Kim and Rethnam, 1997).
Therefore, using of local hemostatic agents in Group B and C were much better than suturing the liver. Using sutures to control bleeding in liver can cause more injuries, both parenchymal and ischemic, in the normal liver. On the other hand, the liver parenchymal is not a favorable tissue for suturing; and with an inexperienced surgeon, the sutures will increase the incidence of liver parenchyma rupture (Nouri and Sharif, 2015). In current study, the PM examination of dogs in Group (A) showed marked adhesions of the sutured part of the liver with the peritoneum. While in Groups (B) and (C) the adhesion was moderate and light, respectively.
Previous studies have been indicating the usefulness of local hemostatic agents in reducing hemostatic time and patients’ need for blood, which improve the postoperative prognosis (Berrevoet and de Hemptinne, 2007; Nouri and Sharif, 2015; Soltani et al., 2013; Nouri et al., 2015; Jackson, 2001). Some of them require the natural functioning body’s homeostatic system to provide a substrate for endogenous coagulation as Gelatin sponge (Jenkins and Janda, 1946); others do not as aluminum chloride (Nouri and Sharif, 2014).
The hemostatic effect of Aluminum Chloride on hepatic bleeding was tested before by (Nouri and Sharif, 2014) they reported that aluminum chloride were effective hemostatic agents in liver bleeding. Similarly, results were obtained in current study showed that aluminum chloride was superior to Gelatin Sponge in hemostasis time, pathological results, and postoperative adhesion. This may be due to acidic properties that help in coagulation of blood protein and closing the opening of blood capillaries rabidly (Porte et al., 1989). This property makes aluminum chloride a highly efficient local hemostatic agent in controlling liver bleeding specially in liver cirrhosis patients (Nouri and Sharif, 2014; Heaton, 2005; Nouri et al., 2014).
Histologically, the suturing technique usually associated with parenchymal injury and post-operative risk such as rebleeding and abdominal compartment syndrome (Nouri et al., 2015). Therefore, various necrobiotic changes were observed which ranged from fatty change to hepatic necrosis. The persistent irritant effect of suture material evoked a pronounced inflammation associated with subsequent periportal and subcapsular fibrosis.
Gelatin sponge mostly depends upon the activation of clot formation (Paulson et al., 2000). The gelatin remnants can induce a foreign body reaction, chronic inflammation, or infection leading to formation of granuloma and interfere with the wound healing process (Samudrala, 2008). (Kang et al., 2012) found that gelatin delays wound healing. Herein; we noticed a marked fibrin deposition within the clot accompanied over granulation tissue formation. The irritant was noticeable especially on the adjacent hepatocytes that separated with inflammatory zone.
Aluminum Chloride has been investigated to arrest skin hemorrhage through acceleration of clot formation. Aluminum chloride action depends mostly on the coagulation of blood and proteins that efficiently seals the injured blood vessels (Nouri et al., 2015). The liver tissue demonstrated marked healing signs under the protective scab. In Group (C), the inflammatory reactions were minimal in comparison with other techniques that might be due to formation of self-protective scab from the clot. Thus, the inflammation wasn’t clearly observed as in other groups.
Aluminum Chloride, compared to Gelatin Sponge and suturing the liver, controlled bleeding in liver tissue in a lesser time, didn’t interfere with tissue healing, and more effective in preventing postoperative peritoneal adhesions. Therefore, Aluminum Chloride offers an effective local hemostatic action to control bleeding in a liver tissue.
Conflict of Interest
The author declares no conflict of interest related to this report.
Authors’ Contribution
Research planning, surgery, writing, and publishing was carried out by all the authors.
References