Advances in Animal and Veterinary Sciences
Pathologic Study on the Enterohepatic Affections in Chickens at Alexandria Province, Egypt
Nermin Mostafa El-Sayed, Samah Shehata Oda*, Hossam Gaafar Tohamy, El-Sayed Mohammed El-Manakhly
Department of Pathology, Faculty of Veterinary Medicine, Alexandria University, Edfina-Rashid-Behera, Egypt.
Abstract | This study was conducted to determine the pathological findings of intestine and liver of chickens at Alexandria province during the period from February 2015 to March 2016. The livers and intestines were taken from different breeds of 100 chickens (67 White broilers, 19 Saso and 14 Balady) and were investigated for bacteriological, parasitological and pathological examination. The bacteriologic results revealed that E.coli and Salmonella gallinarum were the predominant isolated bacteria from hepatic tissues of all examined breeds. Regarding intestinal samples, E.coli was the main isolated bacteria followed by Clostridium perfringens. Parasitological examination showed that Balady chickens was the most affected breed with coccidiosis followed by Saso and White broilers. Hepatic necrosis was the chief hepatic lesion recorded in White broilers (29.41%), Saso (35.71%) and Balady breeds (30.34%). Moreover, necrotic enteritis was the major intestinal lesion recorded in Saso, Balady and White broilers at frequency of (81.25%), (68.75%), (67.80%), respectively. Finally, it could be concluded that colibacillosis is the most prevalent disease affect different chicken breeds at Alexandria province. In White broilers, Clostridium perfringens is the most bacteria that accompanying necrotic enteritis and coccidiosis was widespread among the chickens of this breed.
Keywords | Liver, Intestine, Broilers, Histopathology
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 02, 2016; Accepted | January 18, 2017; Published | January 22, 2017
*Correspondence | Samah Shehata Oda, Assistant Professor of Pathology, Department of Pathology, Faculty of Veterinary Medicine, Alexandria University, Edfina-Rashid-Behera, Egypt; Email: samahoda@yahoo.com
Citation | El-Sayed NM, Oda SS, Tohamy HG, El-Manakhly MES (2017). Pathologic study on the enterohepatic affections in chickens at Alexandria Province, Egypt. Adv. Anim. Vet. Sci. 5(1): 30-38.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.1.30.38
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 El-Sayed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Poultry represents an essential sector in animal production. In many countries poultry is the principal source of economic and high quality human food. Infectious and non-infectious diseases causing deaths among chicken breeds are thought to be a major constraint for profitable poultry production. The etiology of an enteropathy is a multifactorial, for instance combinations of viruses, bacteria, immunosuppression and mycotoxin intoxication may be implicated (Reynolds, 2003). The digestive tract of the chicken is a major location of potential exposure to pathogens. The enteric damage caused by pathogenic bacteria may result in poor feed conversion efficiency and consequently a reduction in body weight gain in poultry flocks. Additionally, more serious bacterial enteric damages will induce obvious disease and high mortality (Porter, 1998). Colibacillosis is a major infectious disease among poultry of all ages and it has an important economic influence on global poultry production. Colibacillosis economic losses mostly result from mortality and reduction in productivity of the diseased bird. It occurs in acute septicemic form causing death and in subacute form that characterized by pericarditis, airsacculitis and perihepatitis (Calnek et al., 1997). Necrotic enteritis (NE) is a worldwide important welfare and economic poultry disease (Cooper et al., 2009). NE is an enterotoxaemic disease caused by Clostridium perfringens type A and C (Wages and Opengart, 2003; Williams, 2005). In poultry, NE is categorized by severe intestinal necrosis, particularly of the proximal jejunum with subsequent high mortality rate (Long, 1973). Many factors have been recognized to influence the onset of NE including coccidial infection (Palliyeguru et al., 2010). Avian coccidiosis is one of the most important diseases causing serious problems and great economic loss to poultry industry (Jadhav et al., 2011). Wherein, it induces weight loss, reduced feed conversion rate, delayed sexual maturity and reduction of egg production (Lobago et al., 2005). Fowl typhoid is an acute septicemic disease of poultry caused by Salmonella Gallinarum leading to important economic losses due to morbidity, mortality, and reduced growth rate (Priyantha, 2012). The control of fowl typhoid is difficult due to vertical and horizontal transmission. Available data to disease incidence are scarce because many cases are prospective to occur in backyard flocks (Barrow and Freitas-Neto, 2011). Liver is one of the most important organs since it acts as a clearing house for toxic substances that enter the body. Intestinal bacteria can reach the liver through extrahepatic biliary system eliciting cholangiohepatitis (Lovland and Kaldhusdal, 1999), cholangitis and pericholangitis (Cotran et al., 1989). Our study was designed to investigate the pathological findings of intestine and liver of chickens in Alexandria governorate.
Material and methods
Sampling
A total of 100 chickens of different ages, sex and breeds (67 White broilers, 19 Saso and 14 Balady) were used in this study. Birds were gathered randomly from farmers, markets and poultry farms at Alexandria province. The age of the collected birds ranged from 4-45 days old. Liver and intestine samples were collected from clinically diseased birds or freshly dead or sacrificed birds. Samples from each bird were collected in sterile disposable containers then transferred as soon as possible in an ice box to the laboratory of Animal Health Research Institute for bacteriological examination. Moreover, the collected organs were carefully examined for any parasitological affection. Tissue specimens from affected livers and intestines were fixed in 10% buffered formalin for histopathological examination. This study was performed during the period from February 2015 to May 2016.
Bacteriological Examination
Samples were prepared according to Thatcher and Clark (1978). The samples were transferred directly into sterile homogenizer flask containing sterile pepton water (2%) and homogenized for 2 minutes at 3000 rpm and cultivated on solid plate media. A loopful from each homogenized sample was directly inoculated onto MacConkey agar –tryptone soya agar-XLD and eosin –methylene blue agar media also sub culture onto salmonella- shigella agar and XLD media. The plates were incubated at 37ºC for 24-48 hours. The suspected colonies were subjected to purification. The cultures were examined macroscopically and microscopically using Gram’s staining technique. The suspected colonies were picked up and inoculated into broth media then incubated at 37ºC for 12 hours. A loopful was streaked on nutrient agar plates and incubated for 24 hours. The growths were examined for purifying and identifying the cultures characters. The cultures were examined macroscopically and microscopically using Gram’s staining technique. In case of isolated of anaerobic bacteria, intestinal contents after scrapping were inoculated into tubes containing cooked meat medium and incubated in anaerobic jar for 48 hours at 37ºC. Aliquots of 0.1ml were streaked in 5% sheep blood agar supplemented with neomycin sulfate (200ug/ml) (Carter and Cole, 1990). The plates incubated anaerobically at 37ºC for 24 hours then the growth was examined macroscopically and microscopically. All the suspected isolates were subjected to series of chemical tests for identification according to criteria described by Edwards and Ewing (1972).
Parasitological Examination
The fecal samples or intestinal scrapings were taken from each bird and were collected in a beaker. The oocysts were isolated by floatation method in saturated sodium chloride and sugar solution (Bowman, 2003), then examined microscopically.
Table 1: Number and percentage of bacteria isolated from liver and intestine of broiler chickens breeds
|
Breeds |
Number of birds |
Bacteria |
Liver |
Intestine |
||
|
No |
% |
No |
% |
|||
|
White broilers |
67 |
E. coli |
45 |
67.16 |
45 |
67.16 |
|
S. gallinarum |
10 |
14.93 |
10 |
14.93 |
||
|
C. perfringens |
- |
- |
35 |
52.24 |
||
|
P. vulgaris |
- |
- |
9 |
13.43 |
||
|
P. aeruginosa |
- |
- |
4 |
5.97 |
||
|
K. pneumonia |
- |
- |
3 |
4.47 |
||
|
E. aerogenes |
- |
- |
2 |
2.98 |
||
|
Saso |
19 |
E. coli |
12 |
63.15 |
12 |
63.15 |
|
S. gallinarum |
2 |
10.53 |
2 |
10.53 |
||
|
C.perfringens |
- |
- |
9 |
47.37 |
||
|
P. vulgaris |
- |
- |
3 |
15.78 |
||
|
P. aeruginosa |
- |
- |
1 |
5.26 |
||
|
K. pneumoniae |
1 |
5.26 |
||||
|
E. aerogenes |
1 |
5.26 |
||||
|
Balady |
14 |
E. coli |
14 |
100 |
14 |
100 |
|
S. gallinarum |
1 |
7.14 |
1 |
7.14 |
||
|
C. perfringens |
- |
- |
6 |
42.86 |
||
|
P. vulgaris |
- |
- |
1 |
7.14 |
||
Pathological Examination
Shortly after birds sacrification the intestinal tract was opened extending from the duodenum to the rectum including both cecal pouches. The gastrointestinal tract and liver had grossly examined carefully. The observed gross pathological lesions were recorded. Then, tissue specimens were collected from liver and intestine of each bird and rapidly fixed in 10% neutral formalin at least 24 hours. The tissue samples were dehydrated in ascending grades of ethyl alcohol concentration, cleared in xylene, embedded in paraffin wax, sectioned at 4µm thickness and stained with haematoxylin-eosin according to procedures described by Culling (1983).
Results
Bacteriological Results
Table 1 illustrates that E.coli were the predominant isolated bacteria from hepatic tissues of all examined breeds at frequency of (100%) in Balady, (67.16%) in White broilers and (63.15%) in Saso breeds. Salmonella gallinarum was the second bacteria isolated from hepatic tissues of affected birds by the rate of (14.93%) in White broilers, (10.53%) in Saso and (7.14%) in Balady breeds. Regarding intestinal samples, E.coli was the main isolated bacteria followed by Clostridium perfringens (67.16% in White broilers, 63.15 % in Saso and 100% in Balady breeds) and (52.24% in White broilers, 47.37% in Saso and 42.86% in Balady breeds), respectively.
Parasitological Results
As shown in Table 2, Balady chickens breed was the most affected breed with coccidiosis (10/14, 71.43%) followed by Saso breed and White broilers (7/19, 36.84 %) and (14/67, 20.90%), respectively.
Table 2: Number and percentage of enteric coccidiosis in broiler chickens breeds
|
Breeds |
Number of birds |
Enteric Coccidiosis |
|
|
No |
% |
||
|
White broilers |
67 |
14 |
20.90 |
|
Saso |
19 |
7 |
36.84 |
|
Balady |
14 |
10 |
71.43 |
Table 3: Number and percentage of histopathological lesions of the livers of chicken breeds in relation to isolated bacteria
|
Lesions |
Breeds |
||||||||||||||
|
White broiler |
Saso |
Balady |
|||||||||||||
|
Lesion |
Isolates |
Lesion |
Isolates |
Lesion |
Isolates |
||||||||||
|
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
No |
% |
||||
|
Congestion |
22 |
25.88 |
E. coli |
11 |
50 |
2 |
7.14 |
E. coli |
1 |
50 |
7 |
30.43 |
E. coli |
7 |
100 |
|
Salmonella |
5 |
22.73 |
- |
- |
- |
||||||||||
|
none |
6 |
27.27 |
none |
1 |
50 |
||||||||||
|
Hemorrhage |
3 |
3.53 |
E coli |
3 |
100 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Vacuolar degeneration |
4 |
4.71 |
E. coli |
2 |
50 |
2 |
7.14 |
E. coli |
1 |
50 |
2 |
8.70 |
E. coli |
2 |
100 |
|
Salmonella |
1 |
25 |
|||||||||||||
|
none |
1 |
25 |
none |
1 |
50 |
||||||||||
|
Fatty degeneration |
2 |
2.35 |
E. coli |
2 |
100 |
- |
- |
- |
- |
- |
1 |
4.35 |
E. coli |
1 |
100 |
|
cholangiohepatitis |
1 |
1.18 |
E. coli |
1 |
100 |
5 |
17.86 |
E. coli |
2 |
40 |
1 |
4.35 |
|||
|
Salmonella |
1 |
20 |
|||||||||||||
|
none |
2 |
40 |
1 |
4.35 |
E. coli + Salmonella |
1 |
100 |
||||||||
|
Hepatic necrosis |
25 |
29.41 |
E. coli |
17 |
68 |
10 |
35.71 |
E. coli |
8 |
80 |
7 |
30.43 |
E. coli |
6 |
85.71 |
|
Salmonella |
3 |
12 |
Salmonella |
1 |
10 |
Salmonella |
- |
- |
|||||||
|
none |
5 |
30 |
none |
1 |
10 |
E. coli + Salmonella |
1 |
14.28 |
|||||||
|
Periportal fibrosis |
2 |
2.35 |
E. coli |
1 |
50 |
1 |
3.57 |
E. coli |
1 |
100 |
- |
- |
- |
- |
- |
|
Salmonella |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|||
|
E. coli + salmonella |
1 |
50 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|||
|
Fibrinous perihepatitis |
9 |
10.59 |
E. coli |
8 |
88.90 |
- |
- |
- |
- |
- |
1 |
4.35 |
E. coli |
1 |
100 |
|
none |
1 |
11.11 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|||
|
Hepatitis |
17 |
20 |
E. coli |
13 |
76.47 |
8 |
28.57 |
E. coli |
4 |
50 |
4 |
17.39 |
E. coli |
4 |
100 |
|
Salmonella |
2 |
11.76 |
Salmonella |
1 |
12.5 |
||||||||||
|
none |
2 |
11.76 |
none |
3 |
37.5 |
||||||||||
|
Total |
85 |
28 |
23 |
||||||||||||
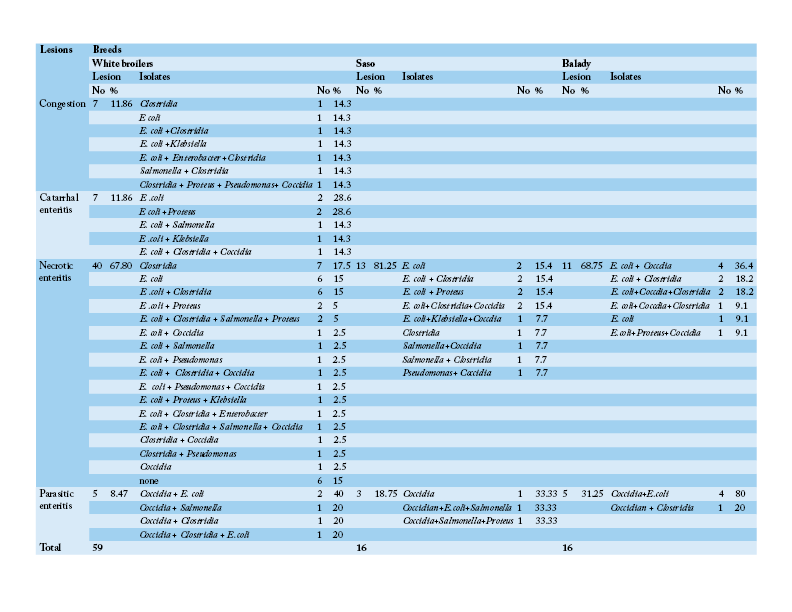
Table 4: Number and percentage of histopathological lesions of the intestine of chicken breeds in relation to isolated bacteria and parasites
Pathological Results
Table 3 shows that the chief hepatic lesions recorded in White broilers breed were hepatic necrosis (29.41%), congestion (25.88%) and hepatitis (20%). However, the predominant hepatic lesions in Saso breed were hepatic necrosis (35.71%) followed by hepatitis (28.57%) and cholangiohepatitis (17.86%). Regarding Balady breed, hepatic necrosis and congestion were recorded as the most prevalent hepatic lesions at frequency of (30.43% for each). Then hepatitis was recorded as the second encountered hepatic lesion (17.39%). Table 4 illustrates that necrotic enteritis was the major intestinal lesion recorded in the three different breeds in Saso, Balady, White broilers breeds at frequency of (81.25%), (68.75%), (67.80%), respectively. Secondly, congestion and catarrhal enteritis were recorded in White broilers at equal frequency of (11.86%). Finally, parasitic enteritis was recorded in Balady chickens at a rate of (31.25%), Saso and white broilers chickens at frequency of (18.75%) and (8.47%), respectively.
Hepatic Lesions
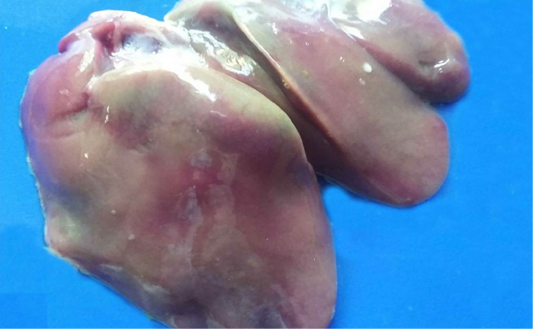
The hepatic lesions detected among the examined chickens were variable. Some livers grossly displayed dark red colour with distended gall bladder and slight enlargement with rounded lobes. Histopathological examination revealed that there were dilatations of sinusoids and portal veins as well as focal areas of extravasations of numerous nucleated erythrocytes (Figure 1A). Other livers were enlarged, pale or yellow in colour, soft in texture (Figure 1B). Microscopically, the hepatocytes were rounded and cytoplasm contained hazy border vacuoles without change in size or location of their nuclei (Figure 1C). Some hepatocytes had tiny sharp outlined vacuoles. Those were progressed to large vacuoles pushing the nucleus to the periphery giving the cells the signet ring appearance (Figure 1D). Liver necrosis was the predominant detectable pathological lesion. At necropsy, these livers had multifocal to diffuse whitish patches (Figure 1E). The microscopic picture was consisted of focal, multifocal to diffuse areas of hepatocytic necrosis. Necrotic hepatocyes were pyknotic with shrunken, fragmented nuclei or even with complete absence of nuclei. These areas were infiltrated with inflammatory cells either heterophils or mononuclear inflammatory cells (Figure 1F). Moreover, livers with bronze discoloration with mottled appearance revealed multiple areas of necrosis and hemorrhage (Figure 2) with characteristic biliary epithelial cells degeneration and necrosis.

Figure 1: Liver of a White broiler chicken A) Infected by E.coli showing extravasations of numerous nucleated erythrocytes (asterisk); B) Infected by E.Coli showing enlargement, yellow in colour and soft in texture; C) Hydropic degeneration of the hepatocytes (arrows); D) infected by E. Coli showing fatty change of the hepatocytes (arrow) and congestion of the blood vessels (blue arrows); E) Infected by Salmonella showing multifocal to diffuse whitish patches (arrows); infected by E. Coli showing Hepatocytic necrosis with inflammatory cells infiltrations (A), H&E. (X250)
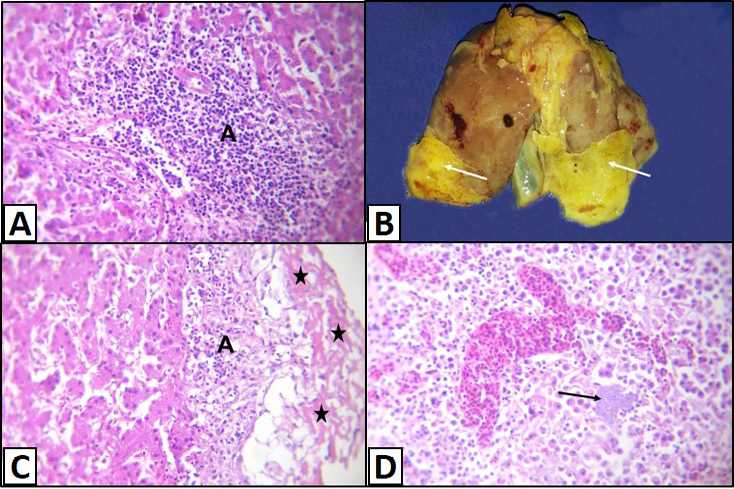
Figure 3: Liver of a White broilers chicken A) Infected by E. coli showing inflammatory cells infiltrations and fibrosis in the portal area (A); B) Infected by E. coli showing grayish whitish fibrin layers (arrows); C) Infected by E. coli showing eosinophilic fibrillar masses (stars) with presence of heterophils infiltration within or under these masses (A); D) Infected by E. coli showing hepatitis that represented by infiltration of inflammatory cells associated with bacterial colonies in the hepatic parenchyma (arrow), H&E. (X250)
Sometimes, the latter lesions were associated with lymphocytic infiltration and fibrosis (Figure 3A). Fibrinous perihepatitis was observed grossly as grayish whitish fibrin layers on the surface of the affected liver (Figure 3B). Microscopically, the covered fibrinous sheet appeared eosinophilic fibrillar masses with presence of inflammatory cells either heterophils or mononuclears infiltration within or under these masses (Figure 3C). Also, fibrinous perihepatitis may or may not be associated with hepatitis that represented by infiltration of inflammatory cells (heterophils and/or mononuclears) associated with bacterial colonies in the hepatic parenchyma or within the portal areas (Figure 3D).
Intestinal Lesions
Different enteric lesions were noticed between the examined chicken breeds. Some intestines were congested and appeared as dark red with presence of hemorrhagic spots (Figure 4A). Microscopically, the affected intestinal blood vessels were dilated and filled with numerous nucleated erythrocytes and may be extravasated from blood vessels in intestinal mucosa, submucosa and tunica muscularis (Figure 4B). Other intestines showed catarrhal enteritis. Macroscopically, there was congestion and the mucosa was covered by translucent viscid mucus. Histopathologically, most of the columnar epithelium lining the intestine changed into goblet cells (Figure 4C). Additionally, there were congested blood vessels with infiltration of inflammatory cells as well as presence of desquamated epithelium inside the intestinal lumen. Necrotic enteritis was observed as the main intestinal lesion in the different examined chicken breeds. At necropsy, intestine was thickened, congested and distended. The intestinal content was dark brown and had necrotic material debris. Microscopically, the mucosa suffered from coagulative necrosis, hyperemic blood vessels and inflammatory cells were seen in the deep layer of the mucosa (Figure 4D). Besides, shortening of villi was observed. Parasitic enteritis was characterized grossly by congested intestine with presence of free blood on mucosa and ballooning of the intestine (Figure 4E). Some cases showed presence of cecal core that was consisted of necrotic debris admixed with blood. Microscopically, there were necrosis and desquamation of epithelial cells as well as hemorrhage in mucosa and submucosa. Moreover, presence of different developmental stages of coccidian parasite was noticed (Figure 4F). In addition, lamina propria and tunica muscularis were infiltrated with inflammatory cells.
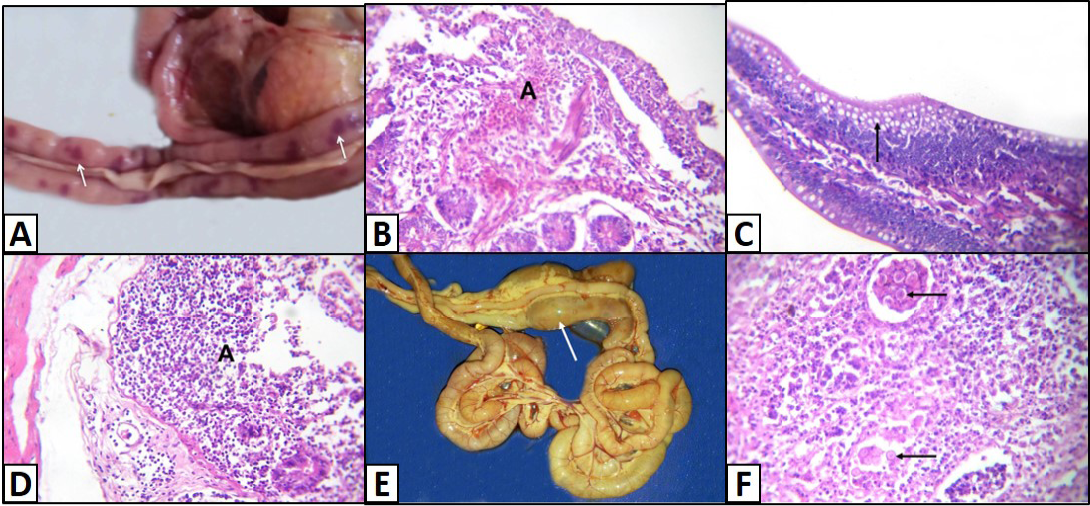
Figure 4: Intestine of A) Saso-breed chicken with coccidiosis showing congestion with multifocal dark red hemorrhagic patches (arrows); B) Balady-breed chicken infected by E. coli and Coccidian spp. showing numerous nucleated erythrocytes extravasated from blood vessels (A); C) White broilers chicken with mixed infection (E. coli and Salmonella) showing hyperplasia of goblet cells (arrow); D) Saso-breed chicken infected by E. coli showing intense inflammatory cells infiltrations in deep layer of the mucosa (A);
Discussion
The enterobacteracae is a group of bacteria including E.Coli, Salmonella, Klebsiella, Proteus which is the most prevalent isolated bacteria from different breeds in Egypt (Khelfa and Morsy, 2015). Collibacillosis is considered to be an important disease affecting the broiler production. Our study revealed that the incidence of collibacillosis was the highest as represented by 100%, 67.16% and 63.15% in Balady, White broilers and Saso breeds, respectively. The gross lesions of collibacillosis in the liver were congestion as well as presence grayish whitish/yellow fibrin layer and pale necrotic foci. Similar findings were described by (Shah et al., 2003). The histopathological lesions were dilatation of hepatic sinusoids, degenerative and necrotic changes in the liver and fibrinous perihepatitis with massive infiltration of inflammatory cells (Gangane et al., 2006). The encountered intestinal lesions of collibacillosis were consisted of congested mucosae that were covered by translucent viscid mucus (Hooda et al., 2011). In addition, the intestinal mucosa suffered from coagulative necrosis, hyperemic blood vessels, massive infiltration of leukocytes and erythrocytes, shortening of villi and goblet cells hyperplasia. Our findings were agreed with (Talha et al., 2001; Islam et al., 2003; Ghosh et al., 2006; Hooda et al., 2011). Regarding to the incidence of Salmonella Gallinarum was 14.93%, 10.53%and 7.14% in White broilers, Saso and Balady breeds, respectively. Livers were enlarged, congested, mottled with presence of necrotic foci. The characteristic bronze discoloration of liver in salmonellosis was identified. Owing to the fact that Salmonella Gallinarum has the predilection for bile canaliculi causing stasis of bile. Microscopically, the affected livers exhibited dilatation of the vasculature, hemorrhage, vacuolar degeneration and focal or massive hepatic necrosis associated with inflammatory cell infiltration either heterophils or mononuclear cells. Similar results were recorded by Sujatha et al. (2003), Basnet et al. (2008), Ahmed et al. (2009), Hooda et al. (2011), Nazir et al. (2012) and Kumari et al. (2013). Additionally, the intestinal mucosa showed congestion, necrotic debris and sometimes covered by translucent viscid mucus. Microscopically, goblet cells hyperplasia and necrotic enteritis were consistent (Sujatha et al., 2003; Freitas Neto et al., 2007; Garcia et al., 2010; Hooda et al., 2011; Nazir et al., 2012; Kumari et al., 2013). Clostridium perfringens are normal inhabitants in chicken intestine and the disease occur when any microbes contribute to damage the intestinal mucosa consequently the clostridium proliferates and generates its toxins (Al-Sheikhly and Al-Saieg, 1980; Kondo, 1988). The frequency rate of Clostridium perfringens identified in intestines of collected birds was 52.24%, 47.37% and 42.86% in White broilers, Saso and Balady breeds, respectively. The gross lesions of intestine were congestion, hemorrhagic spots, necrotic enteritis and ballooning of intestine (Ebtehal, 2000; Kaldhusdal et al., 2001; Wages and Opengart, 2003; Hooda et al., 2011). Coccidiosis causes serious problems with huge economic loss to poultry production. The incidence rate of coccidiosis was 71.43%, 20.90% and 36.84% in Balady, white broilers and Saso breeds, respectively. This result was higher than those reported by Al-Gawad et al. (2012) who recorded an incidence rate 21.24% among Balady breed chickens in Egypt. The high incidence of coccidiosis in this study among Balady breed chickens might be due to wet litter, high stocking density and improper cleaning and disinfection of the most farms before entrance of day-old chicks which may contribute for increase the infestation of coccidiosis. At necropsy, the intestinal mucosa showed intense hyperemia, thickening of the intestinal wall with free blood content, ballooning of intestine and cecal core that characterized by necrotic debris admixed with blood. Microscopically, the affected intestines characterized by necrosis, desquamation of epithelial cells, destruction of villi with hemorrhage and presence of different stages of coccidian parasites (Hooda et al., 2011). Desquamation and damage of intestinal epithelium caused by coccidiosis consider the major predisposing factor for enteric diseases especially necrotic enteritis (NE) (Shane et al., 1985; Frame and Bickford, 1986). Such epithelial damage is permitting the infection by Clostridium perfringens to replicate and produce toxin. Moreover, plasma proteins in the lumen of the gut escaped during coccidiosis are considered favorable nutrient substrates for Clostridium perfringens proliferation and production of toxins (Shojadoost et al., 2012). Finally, it could be concluded that colibacillosis is the most prevalent disease affect different chicken breeds at Alexandria province. Clostridium perfringens is the most bacteria that accompanying necrotic enteritis in White broilers. Coccidiosis was mostly widespread in Balady breed. Hence, control of such diseases through immunization and biosecurity procedures should be taken into consideration.
Acknowledgments
The authors gratefully acknowledge the staff members of Pathology Department, Faculty of Veterinary Medicine, Alexandria University.
Conflict of interest
We declare that there is no conflict of interest.
Authors’ contribution
All authors contributed equally to this work and Prof. Dr. El Sayed Mohammed El Manakhly was the supervisor.
References