Advances in Animal and Veterinary Sciences
Research Article
Evaluation of Anticancer, Analgesic and Anti-Inflammatory Activities of the Ethanolic Extract of Lepidium draba Linn. Leaves
Amer Hakeem Chyad
Department of Physiology and Pharmacology, College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | For the past decades, Lepidium Draba L. was used as one of the traditional medicine against various ailments in Iraq. But the scientific basis for such uses remains largely random. Therefore, the present study applied leaf extract of L. draba to evaluate scientifically its anticancer, analgesic and anti-inflammatory activities. The in vitro anticancer procedures were performed against human cancer cell lines (HeLa) by using different concentrations of L. draba extract that were 31.25, 62.5, 125, 250, 500 and 1000 μg/ml with an incubation period of 24, 48 and 72 hours for each. The analgesic activity was evaluated by both hot plate test and formalin test in Swiss Albino mice at the doses 100, 200, 300, 400 and 500 mg /kg body weight and compared with Aspirin (100 mg/kg body weight) as standard drug. The extract was also investigated for its anti-inflammatory activity at the same above mentioned doses using carrageenan induced mice paw edema method. The results revealed the clear cytotoxic activity of L. draba extract on the growth of HeLa cancer cell line and the effect was concentration-dependent. The treatment with 1000 μg/ml after 72 hrs caused highest inhibition in the viability of HeLa cells (57.65%). The extract showed significant (P<0.05) increase in hot plate reaction time and suppressed the licking activity of both phases. In the formalin test also it caused significant decrease (P<0.05) in paw oedema compared to Aspirin. It can be concluded that L. draba extract has a significant anticancer, analgesic, anti-inflammatory activities.
Keywords | Lepidium draba Leaves, Anticancer, Analgesic, Anti-inflammatory
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | January 02, 2017; Accepted | January 08, 2017; Published | January 10, 2017
Correspondence | Amer Hakeem Chyad, Department of Physiology and pharmacology, College of Veterinary Medicine, University of Baghdad, Iraq; Email: amergyad@gmail.com
Citation | Chyad AH (2017). Evaluation of anticancer, analgesic and anti-inflammatory activities of the ethanolic extract of Lepidium draba Linn. leaves. Adv. Anim. Vet. Sci. 5(1): 7-13.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.1.7.13
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Chyad. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The medicinal plants are those which contain substances in their parts that can be used for therapeutic purpose or as a precursor for the synthesis of other useful medicines. In another speaking, these plants possess therapeutic properties or exert beneficial pharmacological effects on the animal body so they are generally designated as “Medicinal Plants”. Although there were no apparent morphological characteristics in the medicinal plants growing with them, yet they possess some special qualities or virtues that make them medicinally important. It was well established that the plants which are naturally synthesize and accumulate some secondary metabolites, like alkaloids, glycosides, tannins, volatiles oils and contain minerals and vitamins, possess medicinal properties. Medicinal plants constitute an important natural wealth for many countries. They play a significant role in providing primary health care services to rural people. They serve as therapeutic agents as well as important raw materials for manufacturing both traditional and modern medicines. Substantial amount of foreign exchange can be earned by exporting medicinal plants to other countries. In this way indigenous medicinal plants play significant role of an economy of a country. Herbal medicine is relatively cheaper than orthodox medicine, as such it is widely accepted among the people and this could be due to the fact that tradomedical practices blend into the sociocultural life of the people. At present, focus on plant research is increased all over the world and large body of evidence has collected to show immense potential of medicinal plants used in various traditional systems. Use of plant based drugs and chemicals for curing various ailments and personal adornment is as old as human civilization (Saidu et al., 2014).
Among the evaluated 350,000 plant species on the planet, just a little rate has been pharmacological explored and along these lines, the division submitted to natural or pharmacological screening is considerably littler (Hostettmann and Marston, 2002). There accessible proof in the writing guessed that anti-inflammatory and pain relieving compounds could be act likewise as hostile to carcinogenic. These compounds could alter the redox environment of carcinogenic cells and in this way changes their conduct (Schafer and Buettner, 2001). The Lepidium draba was named as a class have a place the family Brassicaceae, privately known as white top or hoary cress. The plant is local to western Asia including Iraq and Eastern Europe and as an intrusive species in North America (Parsons and Cuthbertson, 1992).
L. draba leaves and seeds have laxative and expectorant impacts (Ghahreman, 2009). Additionally, L. draba leaves have impact on some Human Pathogenic Bacteria (Al-Marzoqi et al., 2015). It can be found in many parts of Iraq, in fields and nearby water sources and in patio nurseries and uncovered grounds. L. draba can be found in an assortment of soil sorts where moisture is sufficient (Francis and Warwick, 2008). It commonly grows in an extensive variety of bothered environments including cultivated area, rangeland, pastures, along roadsides, waste regions, and is known not flourish in riparian or inundated zones (Scurfield,1962).
To the best of our insight from the literary works, the pharmacological activities of L. draba were not investigated broadly. The targets of the present study were to inspect the anticancer, analgesic and anti-inflammatory activity of L. draba.
Materials And Methods
Collection of Plant Materials
The leaves of Lepidium draba L. were gathered from Al-Jadriya greenery enclosure of Baghdad University, Baghdad, Iraq before blooming in March, 2015. A voucher example of the plant was stored to be recognized and validated at the National Herbarium of Iraq Botany Directorate in Abu-Ghraib .The plant leaves were dried in the shade for a few days at room temperature and afterward pounded as powder and weighed.
Preparation of Extract
Twenty grams of shade-dried powder was filled in the thimble and removed progressively with ethanol dissolvable in Soxhlet apparatus extractor for 24h. The dissolvable extract was concentrated under lessened weight by rotary evaporator at 40°C . The dry extract acquired was kept in a refrigerator at 4°C.
Experimental Animals
Swiss albino mice with body weight ranged from 18 to 24 g were bought from animal house of Sera and Vaccines Institute. They were left for seven days at standard conditions for acclimatization.
Anticancer Activity
Cell growth: A HeLa cell line was benevolently given by ICCMGR. This human cell line was gotten from cervical malignancy cells segregated from Henrietta Lacks, whom she passed on because of growth on October 4, 1951. These cells were dealt with as malignancy cells, as they are slipped from a biopsy taken from an obvious injury on the cervix as a feature of Mrs. Needs’ analysis of disease. A verbal confrontation still proceeds on the characterization of the cells (Kaplan et al., 1989). Both cell lines were refined in RPMI 1640 medium (Bio Whittaker) containing 20% fetal calf serum (FCS), 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin.
Cytotoxicity assay: Cell cultures were directed in micro-titration plate which was presented to a scope of plant extract concentrations focuses amid the log period of development and the impact was resolved after recovery time. The accompanying convention (Freshney, 1994) was 87 performed on L. draba separate. After trypsinization, the cell suspension was seeded in a microtitration plate at 50000 cells/ml RPMI-1640 development medium with serum 5% utilized for seeding. Plates then incubated for 24 hours by utilizing support medium (free of serum), one-fold serial dilutions were readied beginning from 1000 μg/ml finishing with 31.25 μg/ml. After incubation for 24 hrs and when the cells were in the exponential stage, the medium was evacuated and the concentrate included serially over the plates utilizing repeats of four wells for per each concentration. Added crisp growth medium to the control wells. Just 20 μl of every concentration included for every well. 20 μl of the support medium added to every wall of the control group. The times of exposure were 24, 48 and 72 hours. The plates fixed with self glue film then came back to the hatchery at 36.8 ºC. After the end of the exposure time, the medium tapped off and cells in the wells delicately washed by including and evacuating 0.1 ml sterile phosphate cradle saline two times. At last, 50 μl of 0.01% crystal violet color was included. After 20 min the stain was washed tenderly with faucet water for three times. The plates were left until their dryness. The optical thickness of every wall was perused by utilizing micro-ELISA reader at 495 NM transmitting wave length (Mahony et al., 1989). The percentage of inhibition zone was figured by taking after equation (Chiang et al., 2003):

Analgesic Activity
Hot plate test: Forty two albino Swiss mice were utilized to direct this test and isolated arbitrarily into seven gatherings (6 for each). Group 1: Treated orally with refined water just and served as control, Group 2: Treated orally with 50 mg/kg B.W of L. draba extract, Group 3: Treated orally with 100 mg/kg B.W of L. draba extract, Group 4: Treated orally with 200 mg/kg B.W of L. draba extract, Group 5: Treated orally with 300 mg/kg B.W of L. draba remove, Group 6: Treated orally with 400 mg/kg B.W of L. draba extract and Group 7: Treated orally with 10 mg/kg B.W. of diclofenac sodium.
Thirty minutes after administration, every mouse in all gatherings was dropped tenderly on a plate kept up at 55ºC ± 1ºC. This temperature produces two behavioral parts in mice that can be measured as far as their response times, specifically licking and flicking of their paw and jumping (Okokon et al., 2010).
Formalin test: Forty two albino Swiss mice were utilized, isolated arbitrarily into seven gatherings (6 each). No less than 30 min before performing the formalin test, creators of all gatherings got oral treatment as in taking over: Group 1: Treated orally with refined water just and served as control, Group 2: Treated orally with diclofenac sodium at dosage 10 mg/kg B.W., Group 3: Treated orally with (L. draba) remove at dosage 100 mg/Kg B.W., Group 4: Treated orally with (L. draba) remove at dosage 200 mg/Kg B.W., Group 5: Treated orally with (L. draba) remove at dosage 300 mg/Kg B.W., Group 6: Treated orally with (L. draba) remove at dosage 400 mg/Kg B.W. and Group 7: Treated orally with (L. draba) extract at dosage 500 mg/Kg B.W.
Every mouse was put in transport plastic cage and left 5 min before formalin infusion to permit adjustment the new environment (Hunskaar and Hole, 1987). Ten micrometers of 2% formalin were infused S/C into the plantar region of the right paw of every mouse in every gathering. The infusion of weakened formalin created two agony related behavioral segments that can be measured as far as nociceptive reaction to be specific jumping (it is viewed as one pain related behaviors of the formalin model portrayed by spontaneous, fast and brief shaking or lifting of the paw) and licking of the infused paw (Shibata et al., 1989). Measure of fluid arrangement of treatment materials was balanced by weight of every mouse and given orally through a stomach tube.
Anti-Inflammatory Activity
Carrageenan-induced paw edema: Carrageenan-induced paw inflammation was accomplished by strategically portrayed already (Winter et al., 1962). The mice were arbitrarily chosen and separated into three gatherings (6 each). Group 1: Treated orally with refined water just and served as control, Group 2: Treated orally with diclofenac sodium at dosage 10 mg/kg B.W., Group 3: Treated orally with (L. draba) extract at dosage 100 mg/Kg B.W., Group 4: Treated orally with (L. draba) extract at dosage 200 mg/Kg B.W., Group 5: Treated orally with (L. draba) extract at dosage 300 mg/Kg B.W., Group 6: Treated orally with (L. draba) extract at dosage 400 mg/Kg B.W. and Group 7: Treated orally with (L. draba) extract at dosage 500 mg/Kg B.W.
Swiss albino male mice were dealt with orally with typical saline (as a control group), L. draba separate (100, 200, 300, 400, 500 mg/kg B.W.) and diclofenac sodium at dosage 10 mg/kg, 60 min before 0.1 ml 1% carrageenan infusion. Paw volume was measured before and 1, 2, 3, 4 and 5 hrs after the infusion of carrageenan. Estimations of the paw volume were led by electronic caliper. The paw edema volume was dictated by the distinction between the last and initial measured volumes.
Table 1: Growth inhibition percentage of HeLa cells, by the extract of L. draba during three periods of exposure
|
Type of cell line |
Conc. (μg/ml) |
Growth cell inhibition% |
||
|
24 hrs |
48 hrs |
72 hrs |
||
|
HeLa cell line |
31.25 |
--- |
3.92 |
11.63 |
|
62.5 |
0.84 |
9.53 |
20.92* |
|
|
125 |
5.02 |
17.74 |
28.46* |
|
|
250 |
16.39 |
24.33* |
36.91* |
|
|
500 |
20.32* |
30.14* |
49.04* |
|
|
1000 |
25.76* |
37.31* |
57.65* |
|
Statistical Analysis
The data are elucidated as mean ± SEM and were analyzed statistically by two way ANOVA methodology, trailed by LSD test. A distinction was viewed as significant at p<0.05.
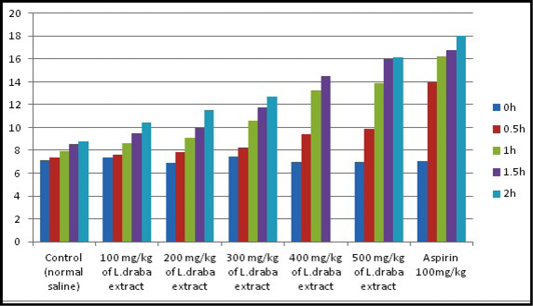
Table 2: Effect of the ethanolic extract of L. draba on latency time in hot plate test
|
Group |
0 h |
0.5 h |
1 h |
1.5 h |
2 h |
|
Control (normal saline) |
7.16±051aA |
7.37±1.16aA |
7.93±0.84aA |
8.57±1.12aA |
8.77±0.29aA |
|
100 mg/kg of L. draba extract |
7.34±1.08aA |
7.59±1.80abA |
8.60±0.32bB |
9.46±1.50bB |
10.39±0.69bB |
|
200 mg/kg of L. draba extract |
6.92±0.66aA |
7.84±1.41bA |
9.11±0.65cB |
9.98±1.28bB |
11.50±0.62cB |
|
300 mg/kg of L. draba extract |
7.41±0.82aA |
8.22±0.96bB |
10.58±0.72cB |
11.72±1.06cB |
12.68±1.13cB |
|
400 mg/kg of L. draba extract |
7.01±1.53aA |
9.37±1.13cB |
13.23±1.10dC |
14.50±0.81dC |
15. 98±1.08dC |
|
500 mg/kg of L. draba extract |
6.98±0.74aA |
9.87±1.66cB |
13.90±0.81dC |
15.95±1.43eC |
16.12 ±0.74deC |
|
Aspirin 100 mg/kg |
7.03±1.13aA |
13.95±0.92dC |
16.23±1.44eC |
16.74±0.80eC |
18.01±1.56eC |
Represent mean ± standard error; Number of group animals, 6; Capital alphabets at same groups mean significant difference (p<0.05) between groups; Small alphabets at same row mean significant difference (p<0.05) between groups
Results
Anticancer Activity
The anticancer activity of the L. draba extract was assessed at 31.25, 62.5,125, 250, 500 and 1000 µg/ml against two malignancy cell lines (HeLa cells). The results were condensed in Table 1. The estimation recorded in this table demonstrated that the growth inhibition was dosage subordinate. Feasibility diminished with expanded time achieving its least impact after 72 hrs of treatment with concentrations utilized. The present records demonstrated most extreme action of the extract (57.65%) at the highest tested concentration (1000 µg/ml). The treatment with 1000 μg/ml after 72 hrs brought about highest reduction in practicality of HeLa cells along these lines achieving 42.35% (percentage of inhibition 57.65%). Further discoveries concerned lower doses of 31. 25 , 62. 5, 125, 250, and 500 µg/ml percent growth inhibition saw by the extract was somewhere around 20.92 and 49.04 % (Table 1).
Analgesic Activity
Hot plate test: Results of the hot plate test are recorded in Table 2. The plant extract showed expansion in the latency time reliant on the dose and the maximum impact was seen with 400 and 500 mg kg at the second hour of study, which was similar to the standard medication (Aspirin 100 mg/kg). As appeared in Figure 1, L. draba extract at 400 mg/kg and 500 mg/kg were showed an advanced increment at the latency time contrasted with the control. The general results were observed to be statistically significant (p<0.05).
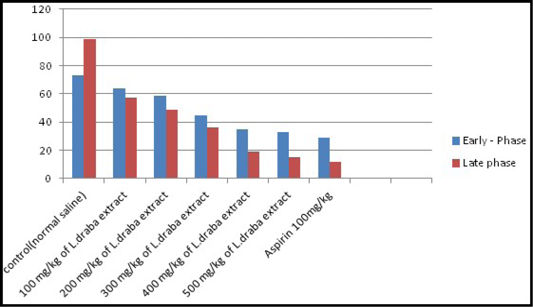
Formalin test: The impacts of the extract of L. draba on formalin induced pain in mice were recorded in Table 3 and clarified in Figure 2. The impact of the extract was dose subordinate. It repressed the licking reaction in both stages and the greatest impacts were seen with 400 and 500 mg/kg at an early stage (35.09 at 400 mg/kg, p<0.05 and 32.82 at 500 mg/kg, p<0.05) and additionally the late stage (19.16 at 400 mg/kg, p<0.05 and 15.22 at 500 mg/kg, p<0.05). The inhibitory impacts were comparable to those of the standard medication (Aspirin 100 mg/kg) which have high decrease results.
Table 3: Effect of L. draba extract on formalin-induced pain
|
Treatment / Dose (mg/kg) |
Early Phase |
Late phase |
|
|
Control (normal saline) |
73.12±2.08aA |
98.72±3.25aA |
|
|
100 mg/kg |
L. draba extract |
64.14±3.16abAB |
57.58±1.79bB |
|
200 mg/kg |
58.35±3.62bB |
48.94±3.12bB |
|
|
300 mg/kg |
44.75±2.89cB |
36.28±2.44cB |
|
|
400 mg/kg |
35.09±1.97cdC |
19.16±2.65dC |
|
|
500 mg/kg |
32.82±2.42dC |
15.22±3.07deC |
|
|
100 mg/kg |
Aspirin |
28.64±2.82eC |
11.95±2.36eC |
Table 4: Anti-inflammatory Effect of L. draba extract on carrageenan-induced paw edema in male mice
|
Treatment group |
0 h |
1 h |
2 hrs |
3 hrs |
4 hrs |
5 hrs |
|
Control (saline) |
2.45±0.32aA |
3.96±0.39aA |
4.97±0.21aA |
5.30±0.28aA |
5.39±0.18aA |
5.42±0.13aA |
|
100 mg/kg of extract |
2.42±0.24aA |
3.87±0.33bAB |
4.11±0.10bB |
4.80±0.31bB |
4.91±0.26bB |
4.90±0.34bB |
|
200 mg/kg of extract |
2.39±0.17aA |
3.82±0.21bB |
3.95±0.12bB |
4.73±0.11bcB |
4.85±0.19cB |
4.83±0.40cB |
|
300 mg/kg of extract |
2.40±0.11aA |
3.77±016aB |
3.89±0.16bB |
4.41±0.29cB |
4.68±0.05dB |
4.59±0.30cdB |
|
400 mg/kg of extract |
2.44±0.29aA |
3.62±0.30cC |
3.75±0.35cC |
4.28±0.36dC |
4.38±0.10eC |
4.32±0.28dC |
|
500 mg/kg of extract |
2.42±0.30aA |
3.59±0.22cC |
3.70±0.29cC |
4.24±0.29deC |
4.35±0.24eC |
4.30±0.21dC |
|
Aspirin 100mg/kg |
2,41±0.16aA |
3.35±0.27cC |
3.49±0.33deCD |
4.10±0.2eD |
4.19±0.11fD |
4.15±0.19eD |
For abbreviations see Table 2
Anti Inflammatory Activity
Carrageenan-induced paw edema: Significant (p<0.05) concealment on carrageenan-induced paw edema was seen after L. draba treatment, while Aspirin (100 mg/kg) indicated higher diminished against the carrageenan-induced paw edema in mice (Table 4, Figure 3).
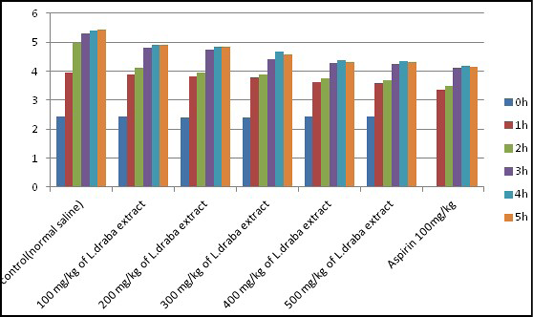
Figure 3: Anti-inflammatory effect of L. draba extract on carrageenan-induced paw edema in male mice
Discussion
There were a few works carrying out to investigate the scientific medicinal value of L. draba. In the present study anticancer action of L. Draba showed diverse Cytotoxicity in vitro against malignant cell lines (L20B and RD) as per its concentration. Already one study existed on the Phytochemistry of L. draba uncovered the nearness of a few basic synthetic gatherings that were alkaloids, terpenoids, tannins, saponins, Leuco anthocyans, triterpenoids and flavonoids. These substances showed obvious inhibitory impacts and have properties to prompt apoptosis on different sorts of growths (Bicha et al., 2016; Shaban et al., 2009). It accounted for that tannin compounds promoted apoptosis and stop one of the cell cycle stages (G, S, G2) on disease cells (Keil et al., 2004).
Others alluded this impact to the phenolic compounds usually found in many plants which forces a wide range of natural effects, including impact on cell proliferation, separations and apoptosis (Tatjana et al., 2010). The anticancer mechanism of L. Draba activity was found, prompting apoptosis.
Presently, the pain relieving activity of L. draba was controlled by two unique tests that were hot plate test and formalin test. The hot plate test was utilized for evaluating the focal antinociception effectiveness and was a shortly composed reaction of pain (LeBars et al., 2001; Shibatta et al., 1989; Chapman et al., 1985) and a central model that has a selectivity for opioid-determined analgesics. The L. draba extract revealed significant expansion in the response time to the thermal stimulus contrasted with pretreatment values.
Treatment of mice with L. draba demonstrated a significant (P<0.05) increment in the response time to the thermal stimulus contrasted with pretreatment values. Given L. draba bench has demonstrated clear significant (P<0.05) increment at the painful reactive time.
In the formalin test which have two particular phases, the initial phase is neurogenic and the final is inflammatory phase (Morales et al., 2001; Dubuisson and Dennis, 1977). In the late stage, a few mediators, for example, histamine, kinin, serotonin and prostaglandins are discharged from the harmed cells, which participate in the inflammatory response and can invigorate the nociceptors and induction of pain (Elisabetsky et al., 1995). In this test, the centrally acting medications, for example, opiates inhibited both phases similarly, while peripherally acting medications just inhibited the second stage (Rang et al.,1998). The effect of the L. draba on both periods of formalin test like Aspirin (100 mg/kg) demonstrated that L. draba may have double activity through central and peripheral mechanism (Sabina et al., 2009).
This increment might be because of the activity of some of its compound constituents specifically questing, a bioflavonoid answered to constrict thermal and substance hyperalagesia (Dubuisson and Dennis, 1977). A few flavonoids apply their antinociception by means of opioid receptor stimulation. Additionally flavonoids, a routine which has a place with quercetine group has an inhibitory effect 5-lipoxygenase pathway (the primary pathway for the production of chemical mediators critical in pain and inflammatory processes) (Rajendran et al., 2000). The L. draba treated groups likewise showed significant (P<0.05) increment at the pain, reaction time, which might be because of the action of its chemical constituents including: phenols, glycosides and alkaloids (Javan et al., 1997). In the carrageenan-induced edema, the extract (100 - 500 mg/kg) wielded significant effect on the early phase of inflammation (1-2 hour) demonstrated an impact most likely on histamine, serotonin and kinin that are included in the early phase of carrageenan-induced odema. The extract brought on additional decrease of the later phase of the odema which might be because of its capacity to inhibit prostaglandin, which is known to mediate the second period of carrageenan induced inflammation (Vane and Booting, 1987). Essentially, Aspirin (100 mg/kg) (a a nonsteroidal anti-inflammatory drug having pain relieving, antipyretic and anti-inflammatory effects by reduction of prostaglandin production by means of cyclooxygenase pathway) whose mechanism of activity includes inhibition of prostaglandin output an extensive inhibition of the paw swelling prompted via carrageenan infusion (Goel, 1999).
This activity might be because of its phenolic compounds constitution that have antibacterial, antioxidant, anti-inflammatory and scolicidal effects. Quercetin can help with the counteractive action of specific diseases, for example, chronic inflammation, atherosclerosis and tumor (Cartea et al., 2010).
Conclusion
The study demonstrated that the extract of L. draba had anticancer, analgesic and anti-inflammatory activities. In any case, the mechanism and the bioactive principles in charge of these activities stay to be illustrated.
Conflict of Interest
There is no conflict of interest.
Authors’ Contribution
All the authors contributed equally.
References