Advances in Animal and Veterinary Sciences
Research Article
Impact of Some Heavy Metals Toxicity on Behaviour, Biochemical and Histopathological Alterations in Adult Rats
Amira Abd El-Moneum Goma1*, Hossam Gaafar Tohamy2
1Department of Animal Husbandry and Animal Wealth Development, Faculty of Veterinary Medicine, Alexandria University, Edfina, Behira, Egypt; 2Department of pathology Faculty of Veterinary Medicine, Alexandria University, Edfina, Behira, Egypt
.
Abstract | Animal welfare is a matter of societal concern and debate, its improvement should consider those aspects actually affecting animal’s health, through determining their impact on some welfare indicators, so, this study was conducted to investigate the effect of heavy metals toxicity on behavioural, biochemical and histopathological changes in adult rats. Twenty-five Sprague-Dawley adult rats were allotted into five groups, each of 5, two groups (GA & GB) treated with lead acetate (low 1g/l & high 2g/l) and another two (GC & GD) treated with anhydrous aluminium chloride (low 2 g/l & high 3.5 g/l) in drinking water daily for 5 weeks, while, GE (control) received drinking water without any treatment. The findings obtained indicated that feeding time, drinking, cage and total exploration frequencies increased in GB than other groups, although, lying time decreased. However, the movement activities increased in GA than others. On the other hand, glucose level decreased significantly in GB than GD and control. Furthermore, triglycerides and total lipid levels were significantly decreased in all treated groups than control. It was also revealed that the serum ALT increased significantly in GB than other groups while creatinine decreased significantly in both groups (GA & GB) treated with lead acetate than other groups with most decrement in high dose group of lead acetate. However, the urea level decreased and uric acid increased significantly in all treated groups than control. Microscopically, degenerated and necrotic hepatocytes, tubular and glomerulus’s necrosis were observed in all toxicated rats beside eosinophilic intranuclear inclusions in lining epithelium of some renal tubules of lead toxicated rats. Therefore, it can be concluded that different toxic levels of lead acetate and aluminum chloride can affect animal’s welfare by behavioural, biochemical and histopathological alterations in a dose dependent manner.
Keywords | Aluminium chloride, Behaviour, Biochemical, Histopathological changes, Lead acetate, Welfare
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 7, 2016; Accepted | August 27, 2016; Published | September 28, 2016
*Correspondence | Amira Abd El-Moneum Goma, Department of Animal Husbandry and Animal Wealth Development, Faculty of Veterinary Medicine, Alexandria University, Edfina, Behira, Egypt; Email: amira_al2008@yahoo.com
Citation | Goma AA and Tohamy HG (2016). Impact of some heavy metals toxicity on behaviour, biochemical and histopathological alterations in adult rats. Adv. Anim. Vet. Sci. 4(9): 494-505.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.9.494.505
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Goma and Tohamy. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Animal welfare is an issue not only for veterinary science and animal psychology but also for biochemical, agriculture, zoology and companion animal fields because animal welfare is a socially constructed idea and it is not a permanently fixed idea (Watanabe, 2007). On the other hand, animals are very good indicator of environmental pollution as they inhabit the same space as human and exposed to action of the same pollutants, for that reason it is appropriate and advantageous to evaluate the negative impact of the polluted environment by heavy metals on human health by parallel evaluation of their load on animals (Kozak et al., 2002).
Lead is a one of the major heavy metals known to be toxic to mammals. It had many sources as metallic lead present in storage batteries, paints and rubbish dumps in areas near lead industrial establishments and also from the industrial applications especially the addition of vast quantities of lead as an anti-knock agent in gasoline (Landrigan et al., 2000). Poisoning with lead is associated with behavioural and physiological problems (Sanders et al., 2009) as it has deleterious effects on neurological, haematological, gastrointestinal, reproductive, circulatory and immunological systems (Patrick, 2006).
Aluminum also is the third most prevalent element and approximately 8% of total mineral components in the earth’s crust (Verstraeten et al., 2008). It is broadly distributed in the environment and widely used in daily life causing its easy exposure to animals and human. It gets access through their body via pulmonary and oral route (Domingo et al., 1993). Although only a small portion of aluminum is absorbed by gastrointestinal tract, oral intake represents the route with greatest toxicological implications (Testolin et al., 1996), especially, it is added to drinking water for purification purposes and found in medicines as antacids (Ochmanski and Barabasz, 2000). Furthermore, it was reported that aluminum exposure can lead to impairments in glucose utilization, free-radical-mediated cytotoxicity and lipid peroxidation (Chinoy, 2001).
The liver is the responsible organ for maintaining the body metabolic homeostasis and has been considered as the target organ for the toxic effect of lead (Patra et al., 2001) and aluminum (Bhadauria, 2012). Moreover, Jarrar (2003), Taib et al. (2004), Shelley et al. (2012) and Al-Qayim and Saadoon (2013) stated that it is the largest repository softy tissue for both toxins followed by kidney where a small amount was excreted in urine and that both metals affect many biological activities in these organs at molecular, cellular and intercellular levels which may result in morphological alterations.
The objective of this study was to investigate the effect of lead acetate and aluminum chloride toxicities on welfare aspects through measuring its effect on some welfare indicators as behaviour, biochemical parameters as well as histopathological changes in adult rats.
Materials and Methods
Animals and Experimental Design.
All experimental procedures were conducted in accordance with the Alexandria University Animal Ethics Committee guidelines. This research was conducted with strict rules to preserve and safeguard the animal welfare without subjecting them to any degree of suffering or stress..
Twenty-five Sprague-Dawley adult rats (three months old, 110-130g) were obtained from the Laboratory Animal Breeding Colony of the Animal health research institute, Alexandria University. They were housed in individual plastic cages (37x30x14cm) and fed on ration containing 16.3% crude protein, 6.8% fat and 3.5% crude fiber, adlibitum with water. Natural light-dark cycle was used without any alteration in lighting program. Identification were carried out by temporary marking on tail using different colour paints and remarking was employed at intervals according to diminish of paint.
After an acclimatization period of one week, the rats were randomly assigned into five groups (five rats each), two groups treated with lead acetate which were, GA (low lead acetate), that was given 1g/l lead acetate and GB (high lead acetate), which was treated with 2 g/l lead acetate in drinking water (Wilson et al., 2000). However, the other two groups were treated with anhydrous aluminium chloride, that were GC (low aluminium chloride), which admistered 2 g/l anhydrous aluminum chloride (Gromysz-Kalkowska et al., 2004) and GD (high aluminium chloride) that was given 3.5 g/l anhydrous aluminium chloride in drinking water (Misawa and Shigeta, 1992). The last group was GE (Control) which was given drinking water without any treatment. The application of treatment was continued for five weeks on daily bases.
Behavioural Observations
Focal sample observations were carried out three times weekly, four hours daily within periods of late morning and early afternoon to obtain a twelve hours daylight observation per week. The observed patterns were ingestive, resting, body care, investigatory behaviour and movement activities (Martin and Bateson, 1993).
Biochemical Parameters
A sample of blood was collected from orbital venous plexus of ether anaesthetized rats at the end of study in a tube without anticoagulant that was left for 3 hours at 4oC to clot then centrifuged at 3000 r.p.m for 10 minutes to obtain serum which was kept at -20oC for biochemical analysis, that includes cholesterol (Thomas, 1992), triglycerides (Sarov and Vlaykova 2005), total lipid (Mccann et al., 1995), total protein (Josephson and Gyllensward, 1975), albumin (Gryzunov et al., 2006), globulin calculated by subtraction of albumin from total protein level, albumin/ globulin ratio, glucose (Trinder, 1969), corticosterone (Pfister and Ivinskis, 1983), ALT (Alanine aminotransferase) and AST (Aspartate aminotransferase) according to (Reitman and Frankel, 1957), γ-GT (Gamma glutamyl transaminase) activity (Tietz, 1994), Creatinine (Larsen, 1972), urea (Coulombe and Favreau, 1963) and uric acid (Whitehead et al., 1991).
Histopathological Studies
Specimens from liver and kidney were collected directly after scarification of examined animals. The samples were fixed in 10% neutral buffered formalin, dehydrated in alcohol, cleared in xylene and embedded in paraffin, 4-6 micron thick sections were prepared and stained with haematoxylin and eosin (Bancroft et al., 1996).
Statistical Analysis
All data were analyzed by one-way analysis of variance, proc GLM, using SAS (Statistical Analysis system, version 6, 4th Edition, SAS Institute, Cary, NC. USA.). Data are expressed as means±S.E.M. and P values<0.05 were considered significant in all tests, unless stated otherwise. Analysis of significant main effects of experimental treatment was performed using multiple range comparisons with Duncan multiple range test.
Results
Behavioural Patterns
The obtained data deducted that feeding time increased significantly in GB & GD than others with the highest increment in GB. Moreover, drinking frequency showed significant increase in GB than other groups. However, lying time decreased significantly in GC and GB than other treated groups and control. On the other hand, movement activities increased significantly in GA than other treated groups and control. However, frequencies of cage and total exploration increased significantly in GB than other treated groups. Moreover, standing time, licking, scratching and trough exploration frequencies showed a non-significant effect for all treatments (Table 1).
Biochemical Parameters.
The biochemical parameters shown in Table 2 revealed that, lipid profile indicated a significant decrease in triglycerides and total lipid levels in all treated groups than control ones except GA in total lipid level. Moreover, it can be deducted that lowest level for cholesterol, triglycerides and total lipid were in GC while the highest were for GA. However, protein profile indicated that albumin level increased significantly in GA and GD than control (3.67+0.14 & 3.10+0.10 vs. 2.69+0.14 g/dl) with the highest in GA, in addition, all treated groups showed increased albumin level than control, while all decreased significantly than GA. Furthermore, it was indicated that the highest level of albumin, globulin and total protein were in GA and lowest level in GB treated rats. Moreover, glucose level decreased significantly in GB when compared to control and GD (79.43+11.45 vs. 120.33+15.59 & 128.17+5.91 mg/dl). However, there was a non-significant effect of treatment on cholesterol, total protein, globulin, albumin/ globulin ratio and corticosterone.
The liver functions enzymes indicated that ALT increased significantly in GB than other groups. Moreover, there was a significant increment in the two treated groups of aluminium chloride than control, in addition to a significant dose dependent increase in lead acetate treated groups. However, AST showed a significant increment in GB than GD. Furthermore, GGT in GC decreased significantly than control. On the other hand, the kidney functions enzymes showed that Creatinine level decreased significantly
Table 1: Least square means and their standard error to the effect of lead acetate and aluminium chloride (Alcl3) toxicities on behavioural patterns of adult rats
|
Item |
Treatment |
||||
|
GA |
GB |
GC |
GD |
GE |
|
|
Ingestive behaviour |
|||||
|
Feeding (min/hr) |
7.08+0.49c |
16.70+2.49a |
8.95+0.87bc |
10.75+1.34b |
6.05+0.50c |
|
Drinking (freq/hr) |
0.74+0.08b |
1.53+0.18a |
0.65+0.10b |
0.55+0.07b |
0.71+0.07b |
|
Resting behaviour |
|||||
|
Standing (min/hr) |
0.48+0.09a |
0.68+0.17a |
0.75+0.15a |
0.83+0.17a |
0.68+0.21a |
|
Lying (min/hr) |
37.37+3.22a |
28.10+4.13b |
28.67+4.25b |
37.78+3.16a |
36.53+3.40a |
|
Body care behaviour |
|||||
|
Licking (freq/hr) |
2.75+0.29a |
3.04+0.15a |
2.56+0.21a |
2.00+0.12a |
3.08+0.70a |
|
Scratching (freq/hr) |
2.10+0.18a |
2.58+0.17a |
3.05+0.20a |
2.31+0.17a |
3.12+0.75a |
|
Movement activities (freq/hr) |
2.26+0.38a |
1.78+0.14ab |
1.51+0.06b |
2.05+0.24ab |
1.58+0.20b |
|
Investigatory behaviour |
|||||
|
Cage (freq/hr) |
1.00+0.18b |
1.69+0.13a |
1.08+0.11b |
1.18+0.12ab |
1.49+0.29ab |
|
Trough (freq/hr) |
0.13+0.02a |
0.13+0.02a |
0.11+0.02a |
0.13+0.01a |
0.09+0.02a |
|
Total (freq/hr) |
1.14+0.18b |
1.81+0.13a |
1.18+0.11b |
1.31+0.13ab |
1.58+0.29ab |
Means within the same raw for the same category carry different superscripts are significantly different. GA: low lead acetate treated group; GB: high lead acetate treated group; GC: low aluminium chloride treated group; GD: high aluminium chloride treated group; GE: control group
Table 2: Least square means and their standard error to the effect of lead acetate and aluminium chloride (Alcl3) toxicities on the biochemical parameters of adult rats
|
Item |
Treatment |
||||
|
GA |
GB |
GC |
GD |
GE |
|
|
Lipid profile |
|||||
|
Cholesterol (mg/dl) |
85.64+10.54a |
81.16+5.70a |
73.33+5.17a |
81.43+2.17a |
87.91+5.81a |
|
Triglycerides (mg/dl) |
134.75+8.18b |
113.31+3.54b |
108.36+14.18b |
115.33+2.03b |
174.67+21.39a |
|
Total lipid (mg/dl) |
330.67+28.72ab |
300.00+14.64b |
279.67+22.81b |
290.00+5.77b |
374.67+29.91a |
|
Protein profile |
|||||
|
Total protein (g/dl) |
7.18+0.08a |
5.54+0.32a |
6.36+0.58ab |
6.15+0.33ab |
6.00+0.58ab |
|
Albumin (g/dl) |
3.67+0.14a |
2.82+0.14bc |
2.95+0.01bc |
3.10+0.10b |
2.69+0.14c |
|
Globulin (g/dl) |
3.51+0.08a |
2.72+0.19a |
3.41+0.58a |
3.05+0.23a |
3.31+0.46a |
|
Albumin/Globulin Ratio |
1.05+0.06a |
1.04+0.04a |
0.93+0.18a |
1.02+0.05a |
0.84+0.09a |
|
Carbohydrate profile |
|||||
|
Glucose (mg/dl) |
103.72+11.4ab |
79.43+11.45b |
94.28+10.14ab |
128.17+5.91a |
120.33+15.59a |
|
Hormone |
|||||
|
Corticosterone (ng/ml) |
129.00+3.00a |
127.00+0.50a |
127.00+0.50a |
127.00+0.50a |
120.00+11.46a |
|
Liver functions |
|||||
|
ALT (U/l) |
67.00+2.89c |
109.04+3.91a |
92.00+2.00b |
88.33+3.18b |
67.00+5.00c |
|
AST (U/l) |
20.67+2.33ab |
31.33+2.60a |
26.00+5.13ab |
19.33+2.03b |
30.00+3.00ab |
|
GGT (U/l) |
7.72+1.67ab |
9.26+0.66ab |
7.34+1.39b |
11.19+1.39ab |
12.36+1.54a |
|
Kidney functions |
|||||
|
Creatinine (mg/dl) |
1.76+0.06bc |
1.68+0.06c |
1.85+0.03ab |
1.97+0.05a |
2.01+0.04a |
|
Urea (mg/dl) |
43.31+1.44c |
46.06+1.97bc |
50.28+1.99ab |
45.64+1.55bc |
53.85+2.22a |
|
Uric acid (mg/l) |
127.07+12.72a |
138.83+10.47a |
129.40+10.47a |
143.57+13.26a |
62.53+4.56b |
Means within the same raw for the same category carry different superscripts are significantly different. For abbreviations see Table 1
in two treated groups of lead acetate than other treated groups and control with most decrement in GB. Furthermore, urea level showed significant decrease in all treated groups than control. Moreover, there was a significant decrement in GA than GC. On the other hand, uric acid level increased significantly in all treated groups than control.
Histopathological Studies
Liver of GA exhibited hydropic degeneration which characterized by the cytoplasm been replaced by clear fluids and the nucleus not affected either in shape or location beside loss of cord arrangement of hepatocytes and portal tract with few aggregations of mononuclear inflammatory cells. Moreover, that of GB showed focal necrosis of the hepatocytes beside fatty degeneration which characterized by large empty vacuoles of sharp borders, that replaced almost the cytoplasm and the portal tract with massive aggregation of mononuclear inflammatory cells which scattered in different spaces with biliary hyperplasia (Figure 1). However, the histopathological examination of the liver tissues of GC revealed remarkable degenerative changes represented by diffuse disorganization of the hepatic cords and moderate hydropic degeneration. While, that of GD exhibited multifocal to diffuse hepatocytes necrosis with fragmented nuclei, ghost or pyknotic and finally necrotic hepatocytes infiltrated with inflammatory cell (Figure 2).
The encountered lesions of the kidney of GA rats were congestion of the blood vessel and inter-tubular blood capillaries, cellular debris in the lumen of the renal tubules beside eosinophilic intranuclear inclusions in lining epithelium of renal tubules and glomerulus’s filtrate occupied the space of bowman capsule. Moreover, tubular dilatation, haemorrhage, glomerulus’s filtrate occupied the space of bowman capsule and finally tubular necrosis were observed in rats of GB (Figure 3).
On the other hand, the noticeable lesions in that of GC was tubular dilatation with cellular debris in its lumen; glomerulus’s filtrate occupied the space of bowman capsule and
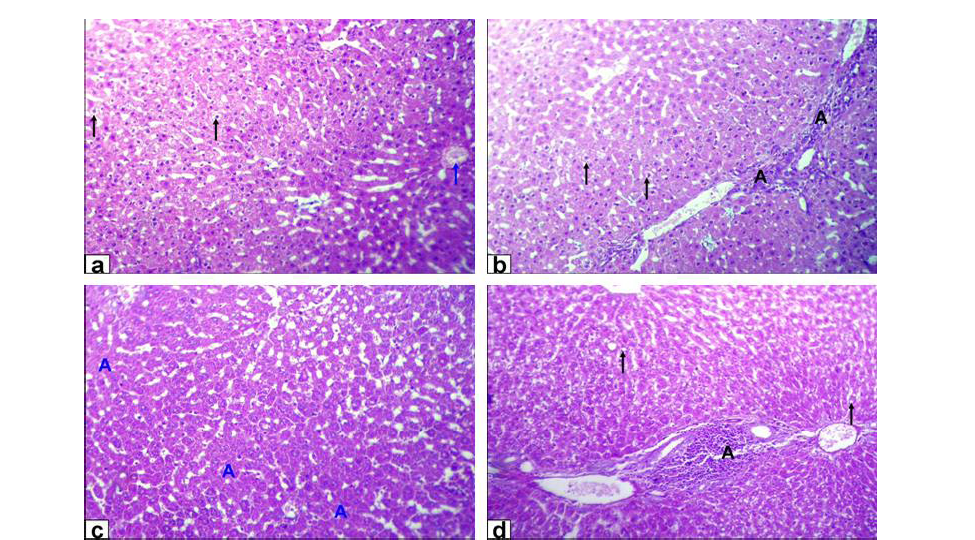
Figure 1: Photomicrographs of liver sections stained with HE from: a and b) Rats treated with low dose of Lead acetate (1g/L) showing hydropic degeneration of the hepatocytes (black arrows), congestion of central vein (blue arrow), loss of cord arrangement of hepatocytes and portal tract with few aggregation of mononuclear inflammatory cells (black A X200); c and d) Rats treated with high dose of Lead acetate (2g/L) showing hepatocytes focal necrosis (blue A) beside fatty degeneration (black arrows) and portal tract with massive aggregation of mononuclear
inflammatory cells which scattered in different spaces (black A) with biliary hyperplasia (X200)
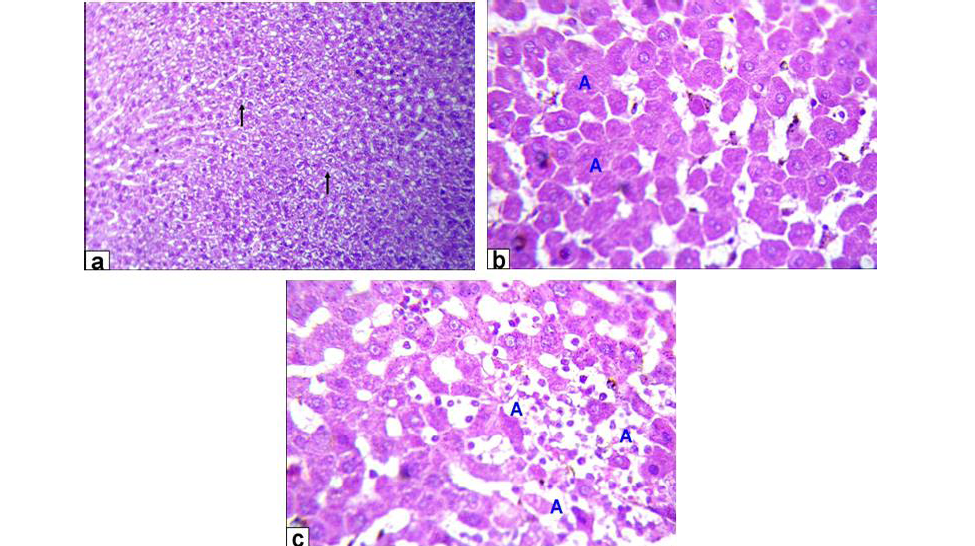
Figure 2: Photomicrographs of liver sections stained with HE from: a) Rats treated with low dose of Aluminium chloride (2g/L) showing moderate hydropic degeneration of the hepatocytes (arrows X200); b and c) Rats treated with high dose of Aluminium chloride (3.5g/L) showing hepatocytes necrosis with nuclei was fragmented, ghost or pyknotic and finally necrotic hepatocytes infiltrated with inflammatory cell (blue A, X 400)
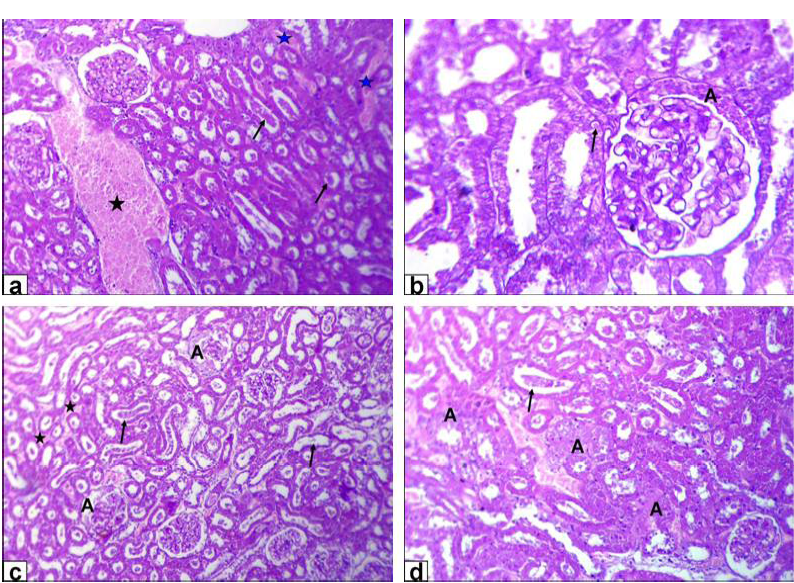
Figure 3: Photomicrographs of kidney sections stained with HE from: a and b) Rats treated with low dose of Lead acetate (1g/L) showing congestion of the blood vessel (black star) and inter-tubular blood capillaries (blue stars), cellular debris in the lumen of the renal tubules (arrows, X200) beside eosinophilic intranuclear inclusions in lining epithelium of renal tubules and glomerulus’s filtrate occupied the space of bowman capsule (black A, X400); c and d) Rats treated with high dose of Lead acetate (2g/L) showing tubular dilatation (arrows), haemorrhage
necrosis of glomerulus’s tuft of bowman capsule and necrosis of some renal tubules (Figure 4A). Moreover, the kidney of GD rats showed complete necrosis of glomerulus’s tuft of bowman capsule, interstitial nephritis with inflammatory cell infiltration and tubular necrosis (Figure 4B).
Discussion
The European Welfare Quality Project defined the criteria of animal welfare as it should include good housing, feeding, health and freedom to behave normally (Stafford, 2014). Furthermore, animal behaviour and health are the most effective animal-based indicators, with researchers/advisers scoring the highest means in case of animal behaviour Moreover, good feeding and housing are from the farm level indicators which are also important for welfare improvement (Averós et al., 2013).
Varies neurotransmitters as monoamines, peptides and amino acids controls ingestive behaviour. Many peptides including cholecystokinin, bombesin, calcitonin, corticotrophin-releasing factor, neurotensin and somatostatin had led to a decrease in the feed intake of experimental animals, in contrast, a small number of them increases feed intake. As early as 1924 it had been reported that peripherally administered insulin stimulated feeding in under nourished diabetic children, which appeared to be secondary to the rapid decrease of glucose in blood as if glucose was maintained constant insulin decreased feed intake (Levine et al., 1986). Moreover, Harris et al. (1998) stated that several cerebral structures and neurotransmitters intervene in the regulation of feed intake processes as serotonin and dopamine that could play primordial role in the control of satisfaction.
Thus the increment in feeding time of high lead acetate group might be under insulin effect which led to drop in glucose level which was also clear in this study, so the in
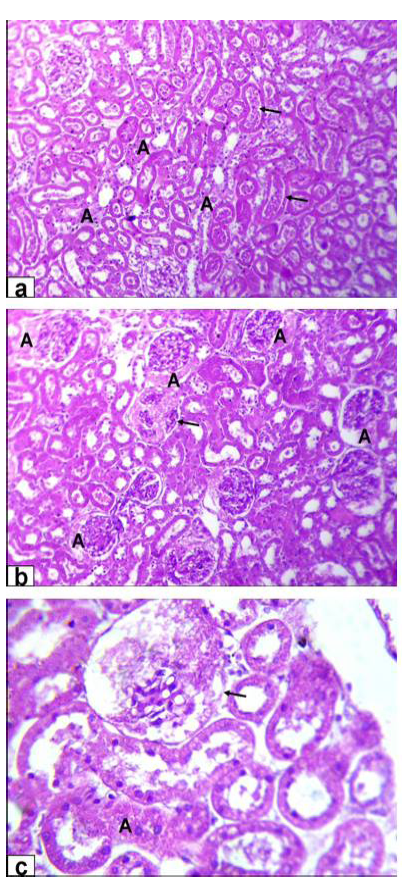
Figure 4A: Photomicrographs of kidney sections stained with HE from rats treated with low dose of Aluminium chloride (2g/L); (a) tubular dilatation with cellular debris in its lumen (arrows) & tubular necrosis (A, X200); b) glomerulus’s filtrate occupied the space of bowman capsule (A) & necrosis of glomerulus’s tuft of bowman capsule (arrow, X200); c) necrosis of some renal tubules (A) and glomerulus’s tuft of bowman capsule (arrow, X200)
crease in feeding time thus correlated by drinking frequency increase. Furthermore, this increase in drinking could be explained by that brain controls many aspects related to the homeostatic processes so the fluctuation in these aspects led the brain to trigger regulatory mechanisms
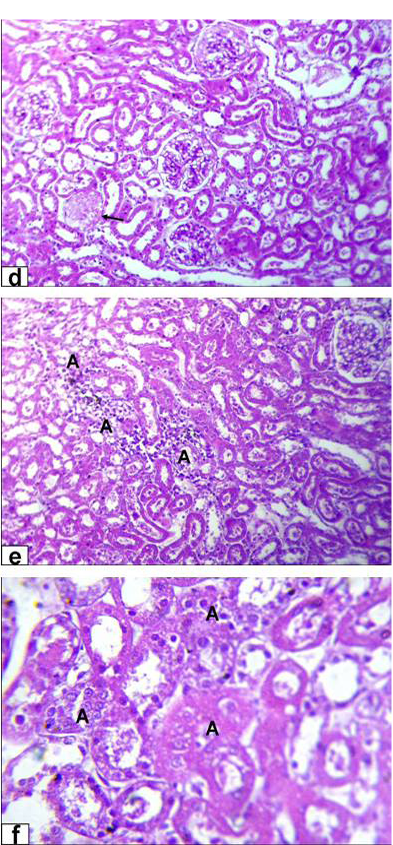
Figure 4B: Photomicrographs of kidney sections stained with HE from rats treated with high dose of Aluminium chloride (3.5g/L) showing (d) complete necrosis of glomerulus’s tuft of bowman capsule (arrow, X200); e) interstitial nephritis with inflammatory cell infiltration (A, X200); f) tubular necrosis (A, X400)
that include renal water and electrolyte excretion and sodium appetite, the vicinity of the third ventricle regions are especially involved in these homeostatic procedures (Johnson and Thunhorst, 1997).
Similarly, Minnema and Hammond (1994) stated that although exposure to lead initially resulted in decreased
nocturnal meal size and duration towards the end of the study, the lead exposed rats had compensated to this by increasing these two parameters during the light phase. Furthermore, the water intake of aluminium treated rats was not different from controls as demonstrated by Mahieu et al. (2009). On contrary, Benlahcen et al. (2009), Kahloula et al. (2009) and Kowalczyk et al. (2004) found a decrease in feed and water intake due to lead acetate and aluminium chloride administration when compared to control group.
The hyperactivity observed in low lead acetate group could be contributed to the hypothesis of dual aminergic and cholinergic neurotransmitters alterations, as the higher aminergic activity, the lower cholinergic activity inducing hyperactivity (Goldberg and Hanin, 1976). Moreover, Luthamn et al. (1994) suggested that changes in motor skills and exploratory behaviour could be induced by lead, which might be in relation to altered dopamine neurotransmitter. These was in consistent with Tawari-Fufeyin et al. (2008) who indicated that guinea pigs intoxicated with lead showed periods of hyperactivity, with restless movements within the cages and in efforts to escape through the mesh holes of their individual cages could have been as a consequence of neurotoxicity of the metal. However, Roig et al. (2006) reported that exposing rats to aluminium in drinking water did not alter significantly motor activity in the open field test. On contrary, Kaoud et al. (2008) showed that lead acetate caused hypoactivity. However, Amit et al. (2012) reported that aluminum exposed mice and rats were more active in the locomotor activity.
The increment of investigatory behaviour in the high lead acetate group are in consistency with Donald et al. (1987) who reported that pups born for pregnant mice administered lead acetate had increased exploratory and social investigation at age of 3-4 weeks than controls. However, Seddik et al. (2010) found that lead acetate administration to rats caused decrease in exploratory activity when compared to control group. Furthermore, Amira and Mahrous (2013b) reported that there was non-significant effect of either metal on investigatory behaviour.
The decrease in lying time of high lead group could be explained by the engage of rats in other behaviours as ingestive and investigatory behaviour to cope with changes present. Similarly, Amira and Mahrous (2013a) and (2013b) reported that in case of lead treated rats there was a decrease in lying time in a dose dependent manner, while, in that of aluminium chloride the decrease was in low dose group only.
Lead and aluminum aggregation in liver and kidney produces toxic remnants by altering various biochemical which including a number of enzymes and cytostructural elements. Its deleterious effects are thought to be mediated via damage to the cell membranes, lipid peroxidation resulting in the loss of membrane fluidity, change membrane potential and/or increase membrane permeability (Lavicoli et al., 2003).
In the present work the lipid profile indicated a significant decrease in triglycerides and total lipid levels in all treated groups. These findings were in parallel with those mentioned by Yousef (2004) and El-Nekeety et al. (2009). On contrary, Shrivastava (2011), Bhadauria (2012) showed that triglycerides levels were higher in the lead and aluminum treated group compared to the control group. Moreover, serum glucose level showed a significant decrease in lead treated groups in a dose dependent manner and not affected by aluminum treatment that agrees with Karamala et al. (2011) and Hassan et al. (2008). This hypoglycemia might be due to the dysfunction of liver as the decrease in glucose-6-phosphatase activity indicates decrease degradation of glycogen, which may result in a subsequent decrease in blood glucose level (Gouda et al., 1985). On contrary, Krishna and Ramachandran (2009) and Bhadauria (2012) showed increase in glucose level of lead and aluminum treated rats.
Hepatic cells share in many of metabolic activities and contain a lot of enzymes. Among these, AST, ALT and ALP are commonly employed biological markers for hepatic injury and efficacy of hepatoprotective interventions (Rosser and Gores, 1995). ALT and AST activity are sensitive indicators of hepatic necrosis (Abdel-Wahhab et al., 2007). Moreover, one of the main indicators of hepatotoxicity is the activity of ALT (Yang et al., 2009).
These findings concerning liver enzymes are in agreement with Suradkar et al. (2009) who announced a dose dependent arise in ALT in rats exposed to deionized water with lead acetate. Furthermore, Krishna and Ramachandran (2009), Hassan and Jassim (2010) and Kowalczyk et al. (2004) showed that AST of lead and aluminium treated rats was very much comparable to control group. Moreover, Bhadauria (2012) and Turkez et al. (2010) indicated that ALT was significantly increased after aluminum exposure. In addition, Mahieu et al. (2009) found that the level of GGT in serum of aluminium treated rats was reduced than controls.
These results may indicate degenerative changes and hypo function of liver (Kaplan, 1987) and might also be attributed to the increased cell membrane permeability or cell membrane damage of hepatocytes caused by lead acetate (Kashyap et al., 2009). Aluminum toxicity was associated with various biochemical abnormalities and these could usually be contributed to the release of intracellular constituents into the circulation, following the loss of cell membrane integrity or interference with normal metabolism and function. Thus the alteration in these enzymatic activities may be due to infusion of them from liver cytosol into blood stream and/or liver dysfunction (Shrivastava, 2011). On contrary to this findings, Niu et al. (2009) and Yousef (2004) found lower level of ALT and AST activities with lead acetate and aluminium chloride toxicity, in addition to, an increase in GGT level of the aluminium chloride treated mice (Shati and Alamri, 2010).
Urea and uric acid are the principal waste products of protein catabolism. They are synthesized in liver from ammonia which produced as a result of amino acids de-amination (Young et al., 1972), while, creatinine is the major waste product of creatine metabolism by muscle. In the kidney, creatinine is distilled by the glomerulus and excreted by the tubules and only free Creatinine appears in the blood serum (Baker et al., 1979). Creatinine and urea could be considered as suitable prognostic indicators of renal dysfunction in case of lead and aluminum exposure.
Rumana et al. (2002) found significant decrease in Creatinine and blood urea nitrogen in lead acetate treated rats. Moreover, Kant et al. (2009) cited that blood urea nitrogen level was decreased after exposure to aluminium sulphate. Furthermore, El-Nahal (2010) and Bhadauria (2012) showed that serum uric acid was significantly increased in lead and aluminum treated rats. This increase in uric acid may be contributed to the impairment in kidney functions (El-Demerdash, 2004). Furthermore, Sadhana et al. (2008) showed that aluminum generates oxidative stress in kidney which caused significantly rise in the activity of uric acid. On contrary to this, Karamala et al. (2011), Shrivastava (2011) and Sadhana et al. (2008) reported that lead acetate and aluminum administration resulted in an increase in serum Creatinine and urea.
The histological examination of the liver tissue for lead acetate and aluminum chloride-treated rats showed mild to moderate acute cellular swelling with hydropic degeneration and multifocal hepatic cell necrosis accompanied by lymphocytic infiltration. The severity of these lesions was dose-dependent. Similar lesions were reported by El-Sokkary et al. (2005) and Agarwal and Jain (2011). Lead-induced portal mononuclear inflammatory cells infiltration, cholestasis and biliary hyperplasia which was well-documented by Hamir and Sullivan (1983). However, fatty changes were sporadic and appeared in a few aluminums treated rat’s hepatocytes.
Kidney histological investigation revealed that lead acetate and aluminum chloride exposure caused progressive glomerular and tubular alterations. Those are in agreement with previous investigations done by Lin et al. (1993) and Abo El-Magd et al. (2015). Interstitial, tubular and glomerular damage are also characteristic renal lesions due to lead acetate toxicity. However, Schraishuhn et al. (1992), Jarrar (2003) and Mcgavin and Zachary (2007) stated that tubular changes occur earlier than glomerular and interstitial changes, including development of pathognomonic eosinophilic intranuclear inclusions in the renal tubular epithelium. These findings showed clear renal lesions associated with chronic lead poisoning and characterized by varying degree of damaged glomeruli, and degenerated proximal tubules in all intoxicated rats in the form of intranuclear inclusion formation, glomerulus’s filtrate occupied the space of bowman capsule, tubular dilatation, hemorrhage and tubular necrosis. Regarding to aluminum induced renal damage, there were tubular dilatation with presence of cellular debris within the lumina of the renal tubules, in addition to necrosis of glomerulus’s tuft of bowman capsule, interstitial nephritis with inflammatory cell infiltration and tubular necrosis was also seen.
CONCLUSION
It can be concluded that both metals affect the welfare of animals at the different stages starting from the behavioural alterations as a first trial of animal to cope with these changes reaching to the pathological affection of the main organs in the body so it is important to record this levels of changes for detection of the welfare alteration of animals in response to these environmental pollutions which indeed will be reflected also on the health of human beings.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the workers who participate to accomplish this work.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Authors’ contribution
Amira A Goma conceived the idea, participated in the design of the study and also performed the oral dosing of animals, behavioural observations, biochemical measures, analyze the data and written the paper. Hossam G. Tohamy conceived the idea, participated in the design of the study and performed the histopathogical studies and written the paper. All the auhtors read and approved the manuscript.
REFERENCES





