Advances in Animal and Veterinary Sciences
Research Article
Pathological Changes of Mycobacterium Tuberculosis and Treatments with Alcoholic Extract of Arachis hypogaea and Ethambutol in Mice
Ban Sahib Al-Nasiry1*, Maysoon Sabah Abbas1, Suhair Hussan Al-Qutbi2
1Zoonotic diseases unit, College of Veterinary Medicine; 2College of Pharmacy, Baghdad University, Baghdad, Iraq.
Abstract | The aim of this study is to demonstrate the histopathological changes of alcoholic extract of Arachis hypogaea and ethambutol in increasing the therapeutic efficacy of anti-tuberculosis in treatment of mice infected with mycobacterium tuberculosis. 30 white Swisrland mice of 6-8 weeks age, weighted 20-25gm were used they were randomly divided into 3 groups which infected with mycobacterium tuberculosis (1x108 cfu) , after one month of infection we treated (2) groups: one of them treated with ethambutol & the other treated with ethambutol + alcoholic extract and third group was contral positive . At 60 day post infection all animals were scarified and samples from different organs (liver, lungs, kidneys, spleen, and intestine) were fixed in 10% neutral buffered formalin for histopathological examination. The result of present study revealed no lesion or mild of internal organs of animal that treated with extract + ethambutol comparing with animal group treated with ethambutol only that showed moderate lesion while infected animal group showed sever pathological lesion characterized by granulomatous lesion in liver and lung also congestion with degenerative changes seen in kidney.
Keywords | Mycobacterium Tuberculosis, Alcoholic extract, Arachis hypogaea
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 10, 2016; Accepted | August 22, 2016; Published | September 28, 2016
*Correspondence | Ban S Al-Nasiry, Zoonotic diseases unit, College of Veterinary Medicine, Baghdad University, Baghdad, Iraq; Email: ban.Sahib@yahoo.com
Citation | Al-Nasiry BS, Abbas MS, Al-Qutbi SH (2016). Pathological changes of Mycobacterium Tuberculosis and treatments with alcoholic extract of Arachis hypogaea and ethambutol in mice. Adv. Anim. Vet. Sci. 4(9): 489-493.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.9.489.493
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Al-Nasiry et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Medicinal plants play an important role in the life of people they used these plants in their daily lives in meals &traditional medicine (Aliasghar et al., 2015). Peanut belongs to the Fabaceae family within Fabaceae Faboideae and its fruit is very distinctive grow underground , they consists of chemically Palmatic acid, Oleic acid, proteins and vitamins B1, B2 , B6 and Lecithin as well as phenolic compounds is a strong antioxidant, that prevent the oxidation of lipids or other molecules and inhibition of oxidative chain reaction. The peanuts also contain sugars, dietary fiber, saturated and unsaturated fats and metals (Lee et al., 2007). Tuberculosis is a common and often deadly infectious chronic disease caused by mycobacterium. A third of world’s population are thought to be infected with M. tuberculosis (Kumer et al., 2007; Jasmer et al., 2002). The infection begins when the mycobacterium reach the pulmonary alveoli where they invade and replicate within the endosomes of alveolar macrophages (Schon and Stendahi, 2013; Houben et al. 2006). The infectious dose of tuberculosis is very low and inhaling less than 3 bacteria may cause an infection (Nicas et al., 2005). Effective tuberculosis treatment is difficults due to unusual stracture and chemical composition of mycobacterial cell wall which makes many antibiotics ineffective and hinders the entry of drugs (Brennan and Nikaido, 1995; Acharya and Goldman, 1970).
This microorganism have multidrug resistant (Newton et al., 2009). So there was a trend towards medicinal plants to see active materials and there role in the inhibition of mycobacterium tuberculosis (Lee et al., 2007). One of these plants is peanut (Arachia hypogaea) which is abundance in many countries especially the warm one used as food as well as the effectiveness as antioxidant (Gupta et al., 2009). Peanuts are known to be rich in L-arginine and supplementation of nutrition rich in arginin may shorten duration of treatment as well as increase recovery in tuberculosis patients (Schon et al., 2003), Arginine increase nitrogen dioxide level which has a role in stimulating the body defences, patient show shorter duration of cough, increased weight gain and higher seputum conversion rate, all tuberculosis patients had lower levels of plasma L–arginine than healthy one (Wood et al., 2010). Therefor, the aim of this study is to demonstrate the effect of Arachis hypogaea and ethambutol on mice infected with mycobecterim tuberculosis.
Materials and methods
Bacterial Isolates
Mycobacterium Tuberculosis Isolates were obtained from tuberculosis institute in Baghdad, the biochemical test were done to these Isolates to confirm their diagnosis and identification (Quinn et al., 2004).
Culture Media
Lowenstein media which is a special media for mycobacterium tuberculosis is prepared according to the production manuals (Quinn et al., 2004).
Determination of Peanuts Extract
The plants were first dried and ground to powder, then 100 g of plant soaked in 500 ml of 70% ethanol for 24 hr, then separated using separator funnel, subsequently filtered through Whattman filter paper No. 1 and filtrate dried. The dried extracted was weighted for prepare the stock solution by dissolved 1g of extract in 5ml of distal water to prepare concentration 200 mg / ml. Then the PH was measured & nominate solution using whatman paper 4.5m and saved in the refrigerator until use (Anessing and Perez, 1993).
Determination of Challenge Dose
The preparation of bacterial suspension of the counting was made by using McFarland tubes according to Balasubramanian et al. (1992).
Drug used for Treatment
Treatment commenced 4 weeks after infection period which corresponds to the peak in primary lesion, using ethambutol which prepared in 50% sucrose and estimated according to Smith et al. (1991).
Experimental Design
Thirty white swisrland mice, 6-8 weeks age were randomly divided equally into 3 groups and treated as following:
1. The 1st group (group of infection) were inoculated intra peritonealy with 0.1 ml of bacterial suspension contain 1x108 CFU of mycoloacterium tuberculosis.
2. The 2nd group (group of treatment with ethambutol) was treated as the 1st group but were given 0.1 ml ethambutol orally daily after 30 day post infection.
3. The 3rd group was infected and treated with 0.1 ethambutol and 0.1 ml of alcoholic extract of Arachis hypogaea. All animals of all groups were scarified after 60 days post infection.
Post mortem examination done to all groups and recording to cross lesion and pieces (1 x 1 x 1cm) from internal organs (liver, lungs, kidneys, spleen, intestine) were fixed in 10% normal buffered formaline for 72 hrs. Then used the routen procedure for histopathogical section preparation according to Luna (1968).
Results
Pathological Changes
Congestion of most examined organs was the main gross lesion in both infected and treated groups with multiple focal granulomatous lesion in liver and lungs of infected non treated group.
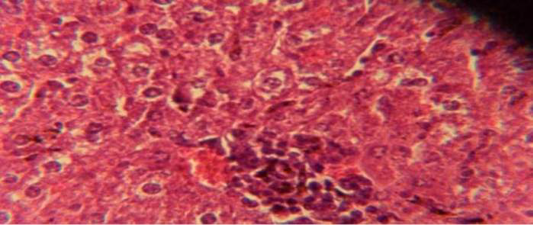
Figure 1: Histological section in liver of infected group showing multiple focal granulamatous lesion in liver parenchyma especially around central vein consist of aggregation of mononuclear cells
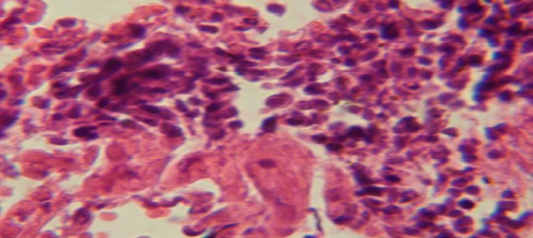
Figure 2: Histological section in lung of infected group showing large granulomatous lesion consist of aggregation of macrophages in lung parenchyma
Histopathological Examination
The liver shows multiple focal granulamatous lesion in parenchyma especially around central vein consist of aggregation of mononuclear cells, with vascular degeneration of hepatocytes (Figure 1) in other animal, the liver shows congestion of central vein with mononuclear cell in their lumen (Figure 2); while, there were aggregation of mononuclaur cell in liver parenchyma and proliferation of kupffer cell (Figure 3).
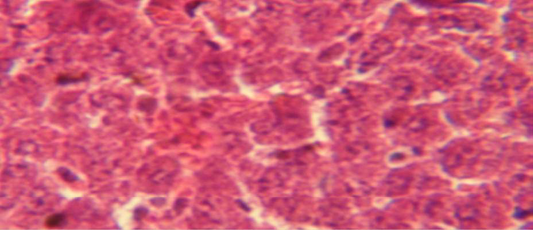
Figure 3: Histological section in liver of treatment group with ethambutol + extract showing aggregation of mononuclaur cell in liver parenchyma and proliferation of kupffer cell
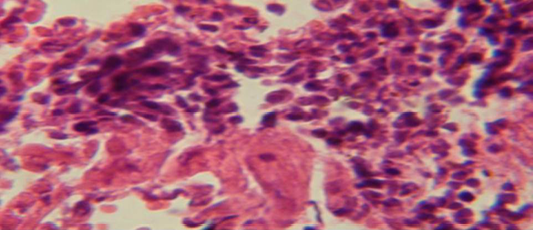
Figure 4: Histological section in lung of infected group showing large granulamatous lesion consist of aggregation of macrophages in lung parenchyma
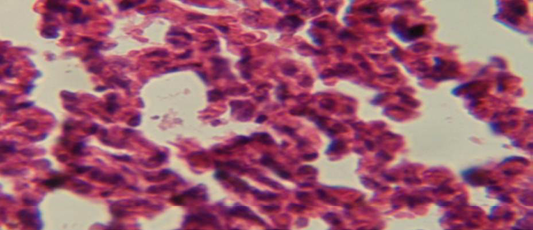
Figure 5: Histological section of lung of treatment group with ethambutol revealed congestion of blood vessel with few inflammatory cell in their lumen
The lung revealed large granulamatous lesion consist of aggregation of macrophages in lung parenchyma (Figure 4), in other animal, there were congestion of blood vessel with few inflamtory cell in their lumen (Figure 5), while Figure 6 shows aggregation of mononuclear cell in the interalveolar septa.
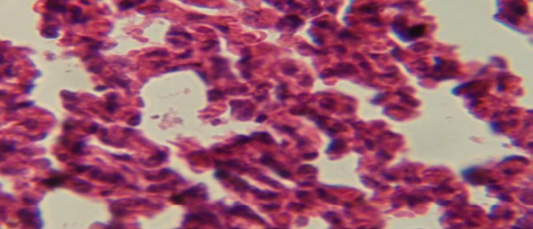
Figure 6: Histological section of lung of treatment group with ethambutol + extract showing aggregation of mononuclear cell in the interalveolar septa
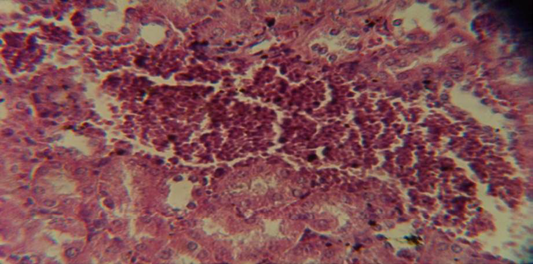
Figure 7: Histological section in kidney of infected group showing congestion of blood vessel with infiltration of inflammatory cell in their lumen
The kidney revealed congestion of blood vessel with infiltration of inflammatory cell in their lumen (Figure 7), in other animal, there were cellular degeneration of epithelial cell lining renal tubule and neutrophil infiltration in the interstitial tissue (Figure 8), while Figure 9 shows cellular degeneration of epithelial cell lining renal tubule.
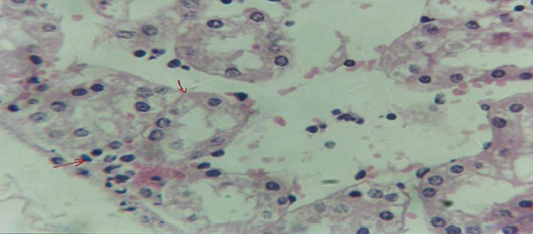
Figure 8: Histological section of kidney of treatment group with ethambutol revealed cellular degeneration of epithelial cell lining renal tubule and neutrophil infiltration in the interstitial tissue
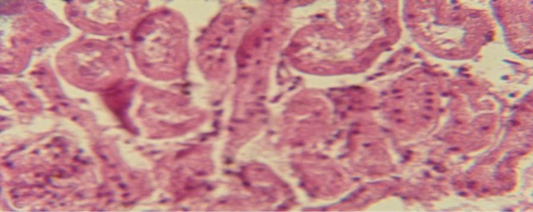
Figure 9: Histological section of kidney of treatment group with ethambutol + extract revealed cellular degeneration of epithelial cell lining renal tubule
Discussion
This study showed the efficiency of alcoholic extract of Arachis hypogaea in treatment of tuberculosis which show no or very less lesion in third group (animals treated with extract + ethambutol) comparing with other groups (Newton et al., 2009) this is usually occur due to high argenine content in extract (Gupta et al., 2009; Abbas, 2011).
Arachis hypogaea enhanced cellular and humoral immune response, also apontent immunostimulator and it is impact may be attributed with flavonoid, proteins and minerals that present in the extract (Nagendra et al., 2015).
Argenine also may play a role in elaborate high percentage of nitrous oxide which consider as effective supporting factor to the activated macrophage (Schon et al., 2003; Wood et al., 2010). The present study shown that intra peritoneal infection does result in chronic TB infection in mice organs similar to that observed during low dose aerosol infection mentioned by (Cardona et al., 1999).
The mycobacterion tuberculosis spread through the blood stream to other tissues and organs and so all parts of body can be affected by the disease (Agarwal et al., 2005) and this agree with our result, that the M.O reach to different organs (lungs, liver, kidneys, intestine and spleen) .
The study revealed that the main histopathological lesion in the examined lungs and livers of inflected animals were granuloma and these observations agreed with (Cardona et al., 2000). The absence of necrosis and langhans type cells makes the tuberculosis granulomas of mice histologically different from those arising in guinea pigs, rabbits and humans, this result supported the investigation that mentioned by (Lefford, 1984). These differential features have been related to the stronger immunological response elicited in mice, which consequently tend to sustain lesser degrees of systemic dissemination of mycobacterial infection than guinea pigs or rabbits (Orme, 1996; Converse, 1996).
Result of present study refers to the effect of arginine in elaborate high percentage of nitrous oxide which support to activated macrophage against mycobaceria. This results agree with Aliasghar et al. (2015), who found that arginine is useful as an adjunctive therapy in patient with active tuberculosis that increased production of nitric oxide and improved the response to treatment.
Virulence is the measure of pathogenicity of microorgansim as determined by it, ability to invade host tissue and produce disease, and after thirty days of infection bacilli had disseminated resulting in microscopically visible lesions in liver, spleen, lungs, these result supported by the observation of Gopinath et al. (2009).
Tuberculosis is classified as one of the granulomatous inflammatory conditions, macrophage, T cell, B cell and fibroblasts are among the cells that aggregate to form granuloma with lymphocytes surrounding the inflected macrophages. T lymphocyte secrete cytokines such as interferon gamma which activate macrophages to destroy bacteria (Kaufman, 2002).
The treatment with ethambutol resulted in significant reduction in pathological lesion in different organs and our result is agreed with Dickinson and Mitchison (1976) who use aguinea pig as amodel of infection.
Ethambutol inhibits arabinosyl transferase-an enzyme that is important for the synthesis of the mycobacterial arabinogalactan cell wall (Richard and Mary, 2006).
Acknowledgments
Many thanks to Zoonotic Diseases Research Unit of the Faculty of Veterinary Medicine/University of Baghdad, for their help.
Conflict of interest
There is no conflict for this research.
Authors’ contribution
Ban AL- Nasiry & Maysson Abbas: collection of human tuberculosis bactrerium and preperation of ethambuttol and Alcoholic extract Peanuts, in addition to the lab work.
Suhair Al-Qutbi: reading the histopathological changes.
References





