Advances in Animal and Veterinary Sciences
Research Article
Haematological Profiles of Olive Baboon, Indian Langur and Hamadryan Baboon in Dhaka Zoo
Muhammad Mustafizur Rahman Chowdhury1, Fahmida Afrin2, Shib Shankar Saha3, Md. Ali Asgar1, Md. Ataur Rahman4, Md. Salim Iqbal5, Md. Moidur Rahman6, M. Bashiruzzaman6, Md. Kamrul Islam6*
1Department of Physiology and Pharmacology, Patuakhali Science and Technology University, Bangladesh; 2Department of Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh-2202; 3Dept of Pathology and Parasitology, Patuakhali Science and Technology University, Bangladesh; 4Department of Surgery & Radiology, Bangabandhu Sheikh Mujibur Rahman Agricultural University,Gazipur-1706, Bangladesh; 5Veterinary Surgeon, Dhaka Zoo, Mirpur, Dhaka, Bangladesh; 6Department of Physiology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202.
Abstract | Haematological parameters with regards to sex and species in Olive baboon (Papio anubis), Indian langur (Presbytis entellus) and Hamadryan baboon (Papio hamadryas) was studied to establish a diagnostic indices for the primates species. The values were obtained from 18 healthy animals of these species in Dhaka Zoo. All the animals were divided into three groups on the basis of species, and categorized into two groups (male and female) on the basis of sex. Sex and species significantly affected the values of TEC, TLC, Hb, PCV, MCV and MCHC among these three different species of primates. Among the three male primates species, Olive baboon, Indian langur and Hamadryan baboon, TEC values was higher in Olive baboon (5.3±0.29 x 106 /µl) compared to others, TLC values was higher in Hamadryan baboon (15±0.89x 103/µl), Hematocrit/PCV was higher in Olive baboon (45±1.9) % and Hb (12.9±0.46 g/dl) was higher in Olive baboon. The average values of RBC among the three female primates species, Olive baboon, Indian langur and Hamadryan baboon, range from 3.62 x 106/µl to 4.48 x 106/µl. The average values of RBC differ significantly between the sexes (P ≤ 0.05). Hb % was 10.46 g/dl to 12.16 g/dl and the values differ significantly between the sexes (P ≤ 0.05). WBC values vary significantly between the two populations (male and female), the percentage of lymphocytes was 57 to 66 % and neutrophils were over 38 to 42% in three species. For eosinophil, monocytes and basophil count, no significant differences were found between the male and female. MCV of male Hamadryan baboon was the highest (85%) compared to the Olive baboon, Indian langur and MCHC values was almost same. MCV of female Hamadryan baboon was the highest (80%) compared to the Olive baboon, Indian langur and MCHC values was almost same. There are no significant differences in between the male and female MCHC values of Olive baboon, Indian langur and Hamadryan baboon.
Keywords | Hematology, Blood, Indian langur, Hamadryan baboon, Olive baboon
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 01, 2016; Accepted | August 10, 2016; Published | August 13, 2016
*Correspondence | Md. Kamrul Islam, Professor, Department of Physiology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202; Email: k_islam88@yahoo.co.in
Citation | Chowdhury MMR, Afrin F, Saha SS, Asgar MA, Rahman MA, Iqbal MS, Rahman MM, Islam MK (2016). Haematological profiles of Olive baboon, Indian langur and Hamadryan baboon in Dhaka zoo. Adv. Anim. Vet. Sci. 4(8): 411-415.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.8.411.415
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Afrin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Wildlife is the integral part of ecosystem and conservation of wild animals has become mandatory for the environment (Meijaard et al., 2006). Growing concern for the world’s endangered wild life has led to addressed conservation action, and legislative bindings have come in action to reserve wildlife in some territory (Jepson et al., 2002). The zoo animal has been used to study the way of disease progresses in wild life (Rust, 2001). Blood is the vehicle for the transport of oxygen and nutrients (Rollins et al., 1990) and knowing of the normal composition of blood and blood products is of prerequisite phenomenon for life. On the basis of normal profiles of blood, the abnormalities related to blood could be easily estimated. The analysis of blood may pave the new way of life by easily diagnosis for wildlife (Weiser, 1987). Patho-physiology of blood is the determinant factor for assessing the condition of the health status of living individual (Kraemer, 1992). Primates could be an important animal for the medical countermeasures against chemical warfare agents (CWAs) (Draghici et al., 2004).
Haematological analyses are used as a valuable tool for evaluating the normal physiological status of wildlife, diagnosing of disease and clinical screening of the patient (Sato et al., 2005). These parameters will provide appropriate reference values for wild species to minimize the special effect of species differences. Sex and age may affect the blood profiles of primates (Ihrig et al., 2001). The establishment of baseline haematological data for animals is of basic importance for. The determination of the standard haematological parameters in primates is important for different scientific investigations (Wallah and Boever, 1983; Melville et al., 1997). It is very much essential to know the normal haematological parameters of wild animal which is hardly available in the literature as these lines have rarely been carried out under local condition. There has been no work on the normal haematological profiles of primates, Olive Baboon, Hamadryan Baboon and Indian langur in Dhaka Zoo. Therefore, the present investigation was initiated to determine the normal haematological values of individual animal, among the species, and between the sexes in Olive Baboon, Hamadryan Baboon and Indian langur.
MATERIALS AND METHODS
Methods
A total of eighteen apparently healthy Olive baboon (Papio anubis), Indian langur (Presbytis entellus), and Hamadryan baboon (Papio hamadryas) average weight 15 kg of 8 to 10 years of age were used in Dhaka Zoo in the January 2012. All the animals were divided equally into 3 according to species and each species were divided on the basis of sex. Total erythrocytes count (TEC), packed cell volume (PCV), haemoglobin (Hb), total leukocyte count (TLC), differential leukocyte count (DLC), mean corpuscular volume (MCV) and mean corpuscular haemoglobin concentration (MCHC) were determined after the blood collection of all groups. Cephalic vein was chosen for the blood collection from the Olive Baboon, Hamadryan Baboon and Indian Langur and then the collected blood was mixed with anticoagulant (4% sodium citrate solution, 1:10). All the haematological tests were done according to the procedure described by Ghai (1999).
Statistical Analysis
The control and treated values were analyzed by SPSS version 18.0 software for windows (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, USA). The mean values were tested by One way ANOVA followed by student t-test. In all the cases, P ≤ 0.05 was reflected significant and the graphical presentation was done by the Sigma Plot version 12.
RESULTS AND DISCUSSION
The blood profiles in case of males were ominously greater (P ≤ 0.05) than in females. In all the cases, we have found the gender variation which are simultaneous to Rovirosa-Hernandez et al. (2012), Chapman and Pavelka (2005), Boere et al. (2005), Aguilar-Cucurachi et al. (2010) and Laboratory Primate Newsletter (2002).
Total Erythrocyte Count (TEC) and Erythrocyte Indices
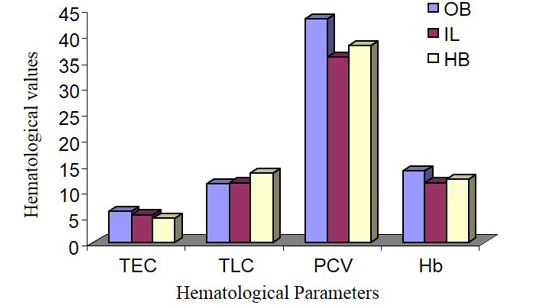
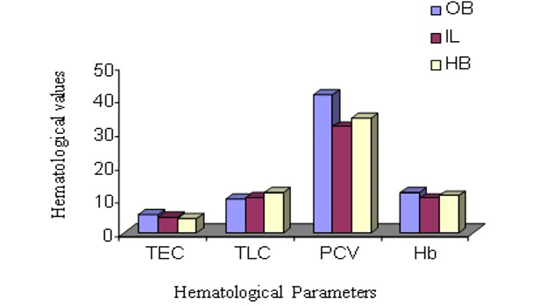
The male animals among different species in this experiment showed the highest TEC value in Olive baboon (Figure 1 and 2), that was suggestively upper (P ≤ 0.05) compared to Indian langur and Hamadryan baboon. In the same way, in female the value was highest in Olive baboon and was observed significantly higher (P ≤ 0.05) TEC value than the other two species. While comparing the results between male and female animals within same species TEC values were meaningfully greater (P ≤ 0.05) in males than in female animals except Hamadryan baboon. This difference of TEC value between the sex may be due to estrogens that have an inhibitory effect on the secretion of erythropoietin (EP), which is the major regulator of erythropoiesis in female, and testosterone in the males that have a stimulatory action on the secretion of EP (Ghai, 1999). The same variation was also recorded for Hb, PCV, MCV and MCHC. The ESR in all these cases followed by inverse relationship with erythrocyte values. The same pattern of results has been documented elsewhere (Mitruka and Rawnsley, 1977).
Total Leukocyte Count (TLC)
The parameters of TLC are shown in Figure 1 and 2. The highest TLC value was observed in Olive baboon and was expressively upper (P ≤ 0.05) than the values obtained in Indian langur and in Hamadryan baboon in the case of both male and female animals. While comparing the values between male and female animals within same species, the TLC values obtained in male animals of Olive baboon, Indian langur and Hamadryan baboon were meaningfully advanced (P ≤ 0.05) than the values obtained in female animals. There is no such hormonal or genetic factor that is responsible for increasing TLC values in male than in females (Rogers et al., 2005), but physiological influences as a behavioural responses may causes an abrupt influence on the increase of leukocyte counts and persuade an inadequate explanation (Jain et al., 1986; Fernie et al., 1994).
Differential Leukocyte Count (DLC)
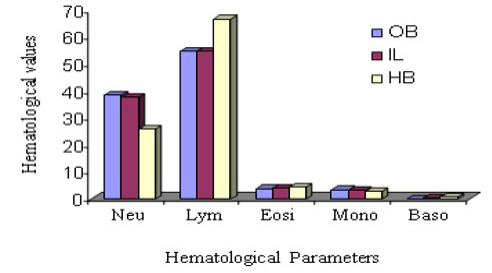
Haematological variation among three species DLC has been stated in Figure 3 and 4. From this observation, neutrophil count was suggestively highest (P ≤ 0.05) in Indian langur than in other two species during comparison of male animals, however, the same parameter in the same species considering the female group. In the same way, lymphocyte count in Hamadryan baboon was pointedly (P ≤ 0.05) higher. In the case of female animals, lymphocyte count was expressively higher (P ≤ 0.05) in Hamadryan baboon compared to the values obtained by Olive baboon and Indian langur. Values of monocyte count in male Olive baboon and in Hamadryan baboon were significantly higher (P ≤ 0.05) than in the same sex of Indian langur, but the values were statistically insignificant in the case of female animals. Neutrophil and lymphocyte count were expressively advanced (P ≤ 0.05) in male animals than in female animals of Olive baboon and Indian langur but higher value in female Hamadryan baboon between the sexes of these species (Figure 3 and 4). Physiological influences as behavioral responses may cause an abrupt impact on leukocyte count and influences inadequate interpretation (Jain et al., 1986; Fernie et al., 1994).
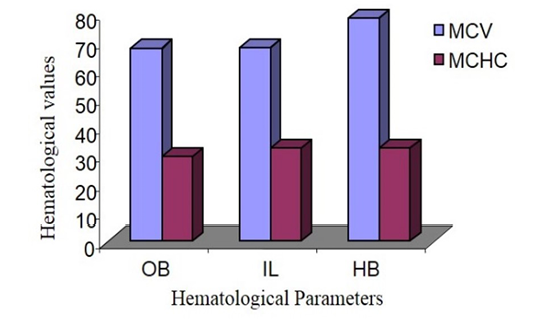
Mean Cell Volume (MCV) and Mean Cell Haemoglobin Concentration (MCHC)
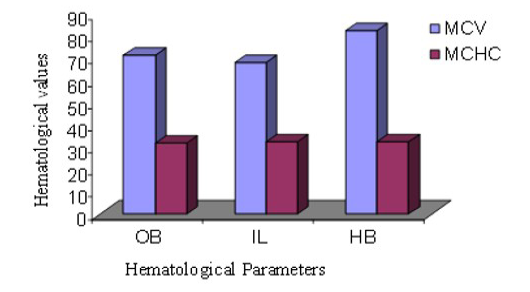
MCV of male Hamadryan baboon was the highest (85%) compared to the Olive baboon, Indian langur and MCHC values was almost same. MCV of female Hamadryan baboon was the highest (80%) compared to the Olive baboon, Indian langur and MCHC values was almost same. There were no substantial dissimilarities in male or female, MCHC values of Olive baboon, Indian langur and Hamadryan Baboon. This was accompanied by Havill et al. (2003) and Bolton (2015), who mentioned that only major sex differences was detected in the haematology of these baboons and that was the lesser glucose levels in females (Figure 5 and 6).
CONCLUSION
The haematological values yielded by the Olive baboon, Indian langur and Hamadryan baboon in the captive condition of tropical weather could be a reference value for biomedical science. In these primates the values of male have been shown to be higher than their female counter parts. Among the three species Olive baboon was the highest in displaying all these haematological parameters. The study reported here was to provide the clinicians and researchers with complete blood profiles for the clinically normal Olive baboon, Indian langur and Hamadryan baboon of different sexes. In addition, these animals can be used as a “disease model animal” of various devastating highly contagious disease like AIDS and their treatment. It may be concluded that these data of zoo animals will provide valuable diagnostic indices for the wildlife which aid them for conservation as well as proper diagnosis and treatments of endangered species and also will provide a clue for the future researcher.
Acknowledgments
The author is grateful to the he author is grateful to the Curettor, Dhaka Zoo for giving the opportunity of this study and also to the BAURES (BAU) for the financial assistance in this research work; Physiology and Pharmacology Department of the Patuakhali Science and Technology University and the Dept of Physiology, Bangladesh Agricultural University for their cooperation in conducting the research work.
Conflict of Interests
The authors have no competing interests.
Authors’ Contribution
M. M. R. Chowdhury designed the experiment and Fahmida Afrin performed the experiment, S. S. Saha analyzes the data, M. M. Rahman performs the hematological studies, Md. Ataur Rahman, M. Bashiruzzaman and Md. Ali Asgar revises the manuscript, M. S. Iqbal helps in the blood collection from the primates and Professor Md. Kamrul Islam supervises as well as helps in writing the manuscript.
REFERENCES
hamadryas). J. Med. Primatol. 32: 131–138. http://dx.doi.org/10.1034/j.1600-0684.2003.00017.x