Advances in Animal and Veterinary Sciences
Short Communication
Case Report on the Treatment of Surgically Debrided Deep Wounds with A New Antioxidant Wound Dressing in Two Dogs
Joan Balasch1, Laura Ramió-Lluch2, Anna Puigdemont3, Begoña Castro4, Felix Bastida5*
1Hospital Veterinari Balmes, Carrer de Balmes, 81, 08008 Barcelona, Spain; 2Laboratorios LETI, Avinguda de Cerdanyola, 92, 08172 Sant Cugat, Barcelona, Spain; 3Universitat Autònoma de Barcelona, Faculty of Veterinary, 08193 Bellaterra, Barcelona, Spain; 4Histocell, Bizkaia Science and Technology Park, 48160 Derio, Bizkaia, Spain; 5Artin Vet Innovative Therapies, Bizkaia Science and Technology Park, 48160 Derio, Bizkaia, Spain.
Abstract | In dogs, large surgical skin wounds are common after tumor resection, trauma or abscesses. In this case report, we present data on the evolution and resolution of two medium-size necrotic wounds of different origin that were treated with surgical debridement followed by a new experimental antioxidant dressing for moist wound care in animals, HR006. Case 1 dog had a deep and wide compression necrosis on the external area of the right hind hock. Case 2 dog had an abscess due to the insertion of plant awns on the right side of the head in the area of the cheek. After systemic antibiotic treatment and surgical debridement, wounds were covered with the experimental antioxidant wound dressing HR006, a matrix of natural galactomannan hydrated with a curcumin and N-acetylcysteine cysteine solution. Dressings were changed 1 to 3 times during the first week, followed by weekly changes for 6 to 7 weeks. In both cases, a healthy granulation tissue appeared very fast that regenerated missing tissue. Re-epithelization borders were also very healthy and quickly covered the affected area. Both cases resolved well within 8 weeks. Moist wound care with an antioxidant dressing for deep and medium-sized wounds produced after surgical debridement and removal of necrotic tissue worked well for prompt evolution and closure of the wounds by second-intention.
Keywords | Surgical debridement, Dog, Moist wound healing, Oxidative stress, Antioxidants
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | April 15, 2016; Accepted | July 04, 2016; Published | July 20, 2016
*Correspondence | Felix Bastida, Artin Vet Innovative Therapies, Bizkaia Science and Technology Park, 48160 Derio, Bizkaia, Spain; Email: bastida@artinvet.com
Citation | Balasch J, Ramió-Lluch L, Puigdemont A, Castro B, Bastida F (2016). Case report on the treatment of surgically debrided deep wounds with a new antioxidant wound dressing in two dogs. Adv. Anim. Vet. Sci. 4(7): 389-393.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.7.389.393
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Balasch et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Skin and soft tissue loss in dogs frequently occur after tumor resections, traffic accidents, hunting accidents or fights. Affected tissues, if not properly cared for, may become infected or necrotic, and deep abscesses can develop. Furthermore, delayed healing and wound chronification can occur (Fahie and Shettko, 2007). Depending on the location, level of tissue loss, destruction or necrosis, surgical removal of the affected tissues will leave mid- to large-sized wounds where border approximation for closure is not an option. In these wounds, secondary or delayed wound closure should be attempted. In addition, delayed closure and open wound management may be indicated when the risk of infection is high (Dernell, 2006).
Moist wound therapy is currently the best option for the care of all types of skin wounds in small animals that cannot be promptly closed surgically (Campbell, 2015). This technique comprises covering the wound with a dressing and proper bandage that will keep moisture at the wound bed and maintain the temperature close to the body’s normal temperature to promote proliferation of fibroblasts, production of extracellular matrix and re-epithelization with keratinocytes that will regenerate the tissues and resolve the lesion (Fahie and Shettko, 2007). However, in the practice of veterinary medicine the use of moist wound care is not as prevalent as in human medicine.
One of the major factors that can delay wound closure is the presence of oxidative stress at the site of injury (Roy et al., 2006). Excess production of Reactive Oxygen Species (ROS)
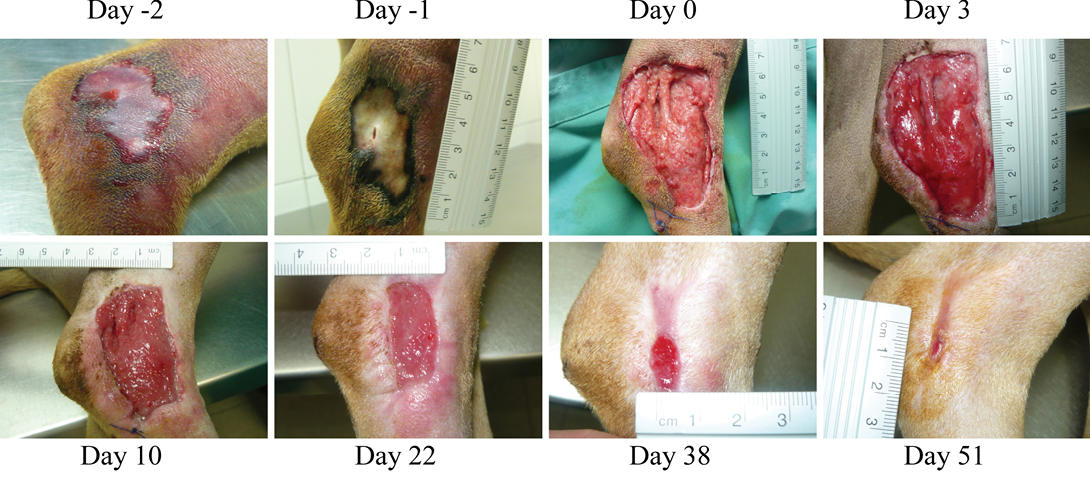
Figure 1: (Case 1)
Necrotic compression lesion on the lateral right hock of a dog. Days were assigned in relation to the application of the antioxidant dressing. Bright red granulation tissue is evident at Day 3. Also, very healthy epithelization borders can be observed quickly after surgery (arrowheads day 3 and 10). These epithelization borders remain healthy and active until closure of the wound
in the wound lead to a pro-inflammatory environment that delays fibroblast migration, neovascularization and the production of granulation tissue, which is essential for re-epithelization and quick wound resolution (Gantwerker and Hom, 2012; Schäfer and Werner, 2008; Stadelmann et al., 1998). In order to address this problem, a new wound dressing with antioxidant properties was developed as previously described (Castro et al., 2015). This new primary dressing has three antioxidant components: a galactomannan of Locust Bean Gum for the preparation of the high absorption capacity matrix, and curcumin and N-acetylcysteine cysteine in the hydration solution. Safety and efficacy studies of this new antioxidant wound dressing using an acute wound model in pigs (excision of the epidermis and dermis) showed that it is safe for animals and efficacious at accelerating wound closure (Castro et al., 2015; dossier for CE mark 0318 medical device class IIb Antioxidant wound dressing). Therefore, we wanted to determine if the antioxidant dressing could also accelerate granulation tissue production and wound closure in dogs with extensive tissue loss after surgical debridement of wounds.
The animals described in this case report were treated at the facilities of Hospital Veterinari Balmes in Barcelona, Spain. Patient owners were informed on the safety and efficacy of the new therapy and gave consent to its use in their animals.
Case 1: A 2-year old female American Staffordshire Terrier of 25 kg was taken to the hospital as a referral case (Figure 1). The animal had previously suffered a traumatic injury on the lateral tarsal joint of the right leg. The injury was treated with a bandage with excessive compression that produced a new lesion. Upon admission at the Balmes Hospital, the patient presented a large necrotic ulcer of approximately 3x6 cm. The wound appeared superficial, was exudative, and no unpleasant smell was detected. The animal had previously been treated with cephalexin, enrofloxacin and firocoxib. After a complete physical examination, treatment with a new antibiotic (cefalotin 22 mg/kg/12h) and NSAIDs (firocoxib 5 mg/kg/24h) was initiated. Also, a proteolytic trypsin and quimiotrypsin based oinment (Dertrase, Laboratorios SALVAT S.A., Barcelona, Spain) was applied on the wound, and the wound was covered with gauze impregnated in a 50% solution of saline and povidone-iodine based product (Braunosan® H plus, B. Braun, Rubi, Barcelona, Spain).
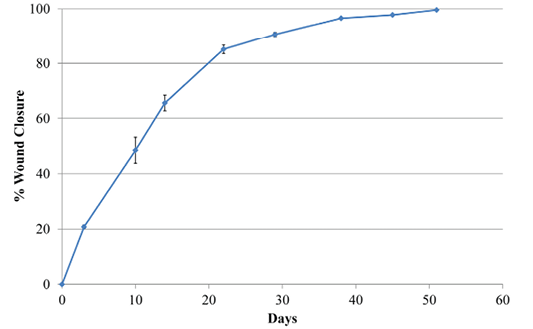
Wound area estimates were calculated with the program Image J (NIH v1.50i) (Rasband, 1997) from different pictures. All pictures had a ruler placed near the lesion. Data were then expressed in relation to the size of the wound at day 0 after surgery and before treatment with the antioxidant wound dressing HR006
Two days later, the dog was sedated and anesthetized (pre-anesthesia: metadone 0.2 mg/kg/iv and acepromacine 0.01 mg/kg/iv; anesthesia: propofol 4 mg/kg/iv), and the wound was surgically debrided. After removal of all necrotic tissues, the wound was covered with the HR006, gauze and elastic bandage. Three days later the wound dressing was changed, and the wound presented a very healthy and clean appearance with no signs of infection (Figure 1). From then on, wound dressings were changed approximately every week, and the new antibiotic and NSAIDs therapies were maintained for 10 days. Wound evolution was notable with a fast production of very healthy granulation tissue. By the end of the second week, granulation tissue had filled most of the cavity, and the area of the wound had decreased by over 50% (Figure 2). Very healthy wound borders were observed without maceration signs and clearly advancing re-epithelization edges (Figure 1 panels Day 10, 22, 38). No signs of infection were observed, and wound closure was obtained after 7 weeks.
Case 2: A 5-year old mixed Griffon female dog of 37 kg with a large necrotic area (approximately 5x6 cm) at the right side of the head (Figure 3) was referred to the hospital after a subcutaneous abscess had burst. The inflammation and necrosis affected the entire area between external ear and eye. Although the dog had been treated with antibiotics and NSAIDs during 35 days, the wound was infected with high levels of purulent exudate. The patient arrived at Balmes Hospital in septic shock with the following parameters: temperature 40°C; glucose 67 mg/dl; systolic BP 80 mmHg; heart rate 140 bpm. The wound was thoroughly cleansed, and fluidotherapy and systemic antibiotics were administered. Over 50 plant awns were removed from all over the body (interdigital space of the front legs; external right ear and oral mucosa) and also from the purulent material in the wound. The dog was hospitalized in the intensive care unit and closely monitored overnight. The next day, after patient stabilization, surgery was performed to remove all damaged and necrotic tissues under sedation and anaesthesia (same as for Case 1). During surgery, multiple plant awns that were producing necrotic foci, were removed from the area of the right masseter muscle. A Penrose drain (Nulife MRK Healthcare Pvt. Ltd. Mumbai, India) was placed in the deepest subcutaneous cavity, and a few approximation sutures were placed around the edges of the wound. The wound was then covered with HR006, followed with a gauze and bandage. Due to the abscess and infection of the wound, the antioxidant dressing was changed at days 3, 4 and 6 during the first week, and then approximately every week. At day 3, a marked improvement of the tissue was observed with new healthy granulation tissue covering the entire wound. A clear reduction in inflammation was evident, and the perilesional tissue appearance had improved. Exudates were still present at several sites but no inhibition of wound healing progression was evident. After granulation tissue had filled in most of the wound cavity at day 6, a remarkable change in the wound was observed by day 13, when a large reduction in the wound area was evident (73% reduction; Figure 3). By day 18, the subcutaneous cavity from the abscess was disappearing, and 10 days later this cavity was practically resolved and re-epithelization phase was progressing properly. The wound closed completely 10 days later.
In this case report, we have shown that the wounds resulting from surgical removal of necrotic tissues after surgical
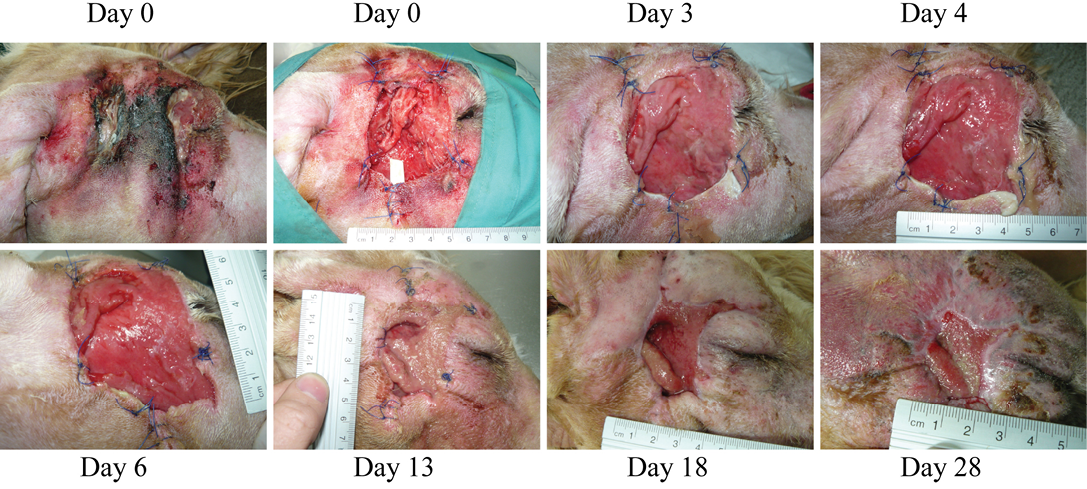
Figure 3: (Case 2)
Necrosis due to foreign body in the right cheek of a dog. At the first wound dressing change, some areas with purulent exudate can still be observed, however, wound progression is evident. Granulation tissue starts quickly filling in the lesion. Dressing change at day 13 was due to removal of the penrose drain and dressing by the patient
debridement can safely and successfully be treated with moist wound therapy with a new antioxidant wound dressing (HR006). Both kinds of wounds described in this case report are of common occurrence in veterinary medicine. Iatrogenic skin and soft tissue injuries caused by inappropriate bandaging have previously been reported both in humans and animals (Anderson and White, 2000). Proper bandaging technique is important in order to prevent these types of lesions (Anderson and White, 2000; Campbell, 2015). Plant awns, particularly from grasses such as foxtail, and spikes and other hard sections of plants, tend to produce lesions in dogs that are sometimes difficult to detect until the condition of the animal has deteriorated significantly due to foreign body abscesses (Vansteenkiste et al., 2014).
One of the properties of moist wound therapy with the new experimental antioxidant wound dressing for animals, HR006, is the neutralization of ROS and thus the detoxification of the exudate resulting in a control of the pro-inflammatory conditions in wounds (Castro et al., 2015). Control of ROS at the wound bed, exerted by the antioxidant components of HR006, may have accounted for the fast production of healthy granulation tissue in the clinical cases described in this case report. Inhibition of the oxidative stress at the trauma site generated by surgical debridement of the wounds may have created the proper conditions for fast neovascularization and fibroblast growth, which are the basis for the production of collagen and other components of the extracellular matrix and for tissue regeneration (Fahie and Shettko, 2007). Large surgical wounds cannot often be resolved by primary intention. Obtaining a healthy granulation tissue in this kind of wound is essential to tissue regeneration and fast wound closure. Indeed in dogs, granulation tissue is not only required for keratinocyte migration and generation of new epithelium, but it is also essential in wound contraction which may account for a wound area reduction of as high as 41% during the first phase of wound healing (Volk and Bohling, 2013).
No unusual scar tissue was observed in either case. A potential problem that sometimes arises when using secondary closure of wounds is the production of excessive scar tissue that may cause functional limitations. Many authors have described that moist wound therapy can prevent or reduce the production of scar tissue (Atiyeh et al., 2003). In both dogs treated with HR006, indirect control of inflammation at the wound bed through the reduction in ROS levels in the exudate may also have contributed to a reduction in scar tissue, since excessive inflammation at the wound site has been reported as one of the leading factors in wound scar formation (Rhett et al., 2008).
Although Case 2 had an abscess and high levels of purulent exudate, no problems with infections or biofilm formation were observed in the wounds of either patient even with spaced out wound dressing changes. Appropriate systemic antibiotic therapy is probably the main reason for this outcome. However, described bacteriostatic effect of some of the components of the new wound dressing (curcumin, N-acetylcysteine cysteine, EDTA) may have contributed to bacterial control and biofilm prevention (Finnegan and Percival, 2015; Packiavathy et al., 2014; Rasmussen et al., 2016). Topical application of antibiotics in wounds is currently controversial, not only because of the potential inhibitory effect on new tissue formation, but also because of the potential generation of resistant bacterial strains due to the fact that the exudate will dilute the antibiotic below the effective bactericidal concentration. For wound treatments, systemic administration of antibiotics, if needed, is recommended because appropriate and continuous levels of antibiotics can be obtained in the wound bed (Campbell, 2015; Fahie and Shettko, 2007). In summary, antioxidant moist wound therapy may be a good alternative for second-intention closure of deep surgical wounds.
Acknowledgements
FB has partial funding from the European Social Fund through a grant of the program Torres Quevedo from the Spanish Ministry of Economy and Competitiveness (PTQ-13-06469).
Conflict of Interests
BC and FB work for the company that developed the antioxidant wound dressing.
Authors’ Contribution
LRL, AP, BC and FB designed the study; JB treated the animals and collected the data, and FB and JB drafted the manuscript. All authors reviewed, corrected and improved the manuscript.
References