Advances in Animal and Veterinary Sciences
Research Article
Investigation on the Distribution of Leptospira Serovars and its Prevalence in Bovine in Konkan Region, Maharashtra, India
Vinayagamurthy Balamurugan*, Sushma Rahim Assadi Thirumalesh, Sudharma Veena, Anusha Alamuri, Mohandoss Nagalingam, Rajangam Sridevi, Gurrappa Naidu Govindaraj, Divakar Hemadri, Mukund Raghavendra Gajendragad, Habibur Rahman
Indian Council of Agricultural Research-National Institute of Veterinary Epidemiology and Disease Informatics (ICAR-NIVEDI), Ramagondanahalli, Post Box No. 6450, Yelahanka, Bengaluru - 560064, Karnataka, India.
Abstract | Leptospirosis is a neglected most widespread re-emerging global zoonotic disease affecting both animals and humans diversely in developing countries. In this study, investigation was carried out to know the distribution of leptospira serovars and its seroprevalence in bovine population in Konkan region of Maharashtra, where human cases of leptospirosis have been reported periodically. A total of 575 serum samples (Cattle-171; Buffaloes-245; Bullocks-81; Bulls-78) randomly collected from coastal areas (Sindhudurg-174, Raigad-90, Thane-230, Mumbai-81) were tested at 1:100 dilution in microscopic agglutination test (MAT) using live antigens of 18 reference leptospira serovars. The overall seroprevalence of 41.04% (236/575) (CI: 95% 37.01 to 45.20) with 34.5% in cattle, 52.2% in buffaloes, 32.1% in bullocks and 29.4% in bulls was observed. The results of chi-square test revealed that seroprevalence is not independent across the age groups for buffaloes (χ2 =9.98, p <0.01), bulls χ2 =21.26, p<0.01) and bullocks χ2 =10.89, p <0.01), but independent in cattle χ2 =4.97, p >0.05). Among the coastal areas, high prevalence was observed in Mumbai (66/81=81.4%) followed by Thane (87/230=37.8%), Sindhudurg (56/174=32.1%) and Raigad (27/90=30.0%). The prevalence is significant and not independent across the species (χ2 = 22.7, p <0.01) and study regions (χ2 =65.90, p <0.01). The predominant leptospira antibodies against major serovars were Australis (23.61%) followed by Hardjo (19.44%), Hebdomadis (16.67%), Bankinang (15.28%), Icterohaemorrhagiae (14.58%), Kaup (9.03%), Tarassovi (7.64%) etc. determined against frequency of distribution of the serovars. This study supports that bovines may have a role in maintaining Australis serovar and some intermediate species serovars, apart from being a well-known reservoir for Hardjo serovar in bovine in Konkan region of Maharashtra state, India.
Keywords | Leptospirosis, Serovars distribution, Prevalence, Maharashtra, Bovine, MAT
Editor | V. Gnanavel, Virology Division, ICAR-Indian Veterinary Research Institute (ICAR-IVRI), Mukteswar, Nainital, Uttarakhand, India.
Special Issue | 2, 2016 “Emerging Challenges and Opportunities in Veterinary Research for Improvement of Animal Health”.
Received | April 01, 2016; Accepted | April 03, 2016; Published | April 20, 2016
*Correspondence | Vinayagamurthy Balamurugan, ICAR-NIVEDI, Post Box No. 6450, Yelahanka, Bengaluru-560 064, Karnataka, India; Email: balavirol@gmail.com; b.vinayagamurthy@icar.gov.in
Citation | Balamurugan V, Thirumalesh SRA, Veena S, Alamuri A, Nagalingam M, Sridevi R, Govindaraj G, Hemadri D, Gajendragad MR, Rahman H (2016). Investigation on the distribution of leptospira serovars and its prevalence in bovine in Konkan region, Maharashtra, India. Adv. Anim. Vet. Sci. 4(2s): 19-26.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.2s.19.26
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Balamurugan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Leptospirosis is a zoonotic disease with ubiquitous distribution, caused by infection with pathogenic leptospira species. It is among the fastest re-emerging anthropozoonosis causing considerable public health problems in most of the countries of Asia, Africa and Latin America driven by climate and environment. Despite being so severe, this disease is neglected in most endemic countries in the world because of the lack of information and awareness about the extent of the problem (WHO, 2011). The disease affects a variety of domestic animals viz. cattle, buffalo, goat, sheep, horse and swine resulting in heavy economic losses to the farming community on account of reproductive problems (Srivastava, 2008). In cattle, leptospirosis causes abortion, infertility, stillbirths, birth of weak calves, reduced productivity and decreased milk yield (Quinn et al., 1994). Serovars causing infection in cattle have been classified into 2 groups: those adapted to and maintained by other cattle, and incidental infections caused by strains maintained by other domestic and free-living animals. Cattle are the maintenance host for serovar Hardjo, which consist of two serologically indistinguishable but genetically distinct species (Ellis, 1994). Besides being an important cause of bovine abortion, reduced fertility and agalactia, serovar Hardjo also poses a potential zoonotic threat to humans who are exposed to infected cattle (Samina et al., 1997).
The leptospirosis situation in India is a cause of concern and it is endemic in all Southern states (Kerala, Tamil Nadu, Andhra Pradesh, Karnataka), other coastal states like Gujarat, Maharashtra, including Andaman and Nicobar Islands of India, where high prevalence was recorded both in animals and humans (Srivastava et al., 1983; Srivastava et al., 2003; Vijayachari et al., 2008). Infection with host-adapted serovars have been reported to produce subclinical infection with apparently healthy animals serving as chronic carriers and persistent shedders of the organism through their urine, body fluid or tissue. In any particular geographical region, various leptospira serovars are prevalent and are associated with one or more maintenance host (s) that serves as reservoir of infection to incidental host or accidental host (human). Moreover, knowledge of the prevalent serovar (s) and their maintenance host (s) in any particular geographical location (s) is essential to understand the epidemiology of leptospirosis and establishing public health policies aimed at its diagnosis and control.
The microscopic agglutination test (MAT), is a well proven, accepted and widely used gold standard serological test for the detection of leptospira antibodies in animals and humans (OIE, 2013). The MAT measures mainly IgM, the titres of which peak after 10 to 20 days but decline within 6 to 12 months and consequently demonstrates recent infection (Pritchard, 2001). Generally, very high MAT titres with consistent clinical features are conclusive of leptospirosis.
Hence, the present study was carried out to determine the frequency distribution of various leptospira serovar representing serogroup specific antibodies and its prevalence in bovine population in Konkan region of Maharashtra state in India.
MATERIALS AND METHODS
Serum Samples
The samples screened were from the coastal areas in Konkan region of Maharashtra. The region lies in the western part of Maharashtra including coastline and adjoining areas comprising of four districts. The latitude and longitude of the region is 18.5 N and 73.15 E. The area is characterized by a tropical climate with average temperature of about 30°C throughout the year and the average rainfall is 3000 mm. A total of 575 bovine serum samples (Cattle-171; Buffaloes-245; Bullocks-81; Bulls-78) randomly collected from different villages/taluk in various areas (Sindhudurg-174, Raigad-90, Thane-230; Mumbai-81) of region by field veterinary officers during different surveys (three from June to August 2012; one from 2013-2014 and 2015-2016 each) were used (Table 1). The sample surveyed places are depicted in GIS Map based on their geo-coordinates using QGIS software version 2.12.3 Lyon (Figure 1). Generally serum samples were received from flood prone area from randomly selected villages/taluk based on the human incidence for diagnosis of leptospirosis. These samples were submitted to ICAR-NIVEDI for serodiagnosis of leptospirosis by Disease Investigation Section, Western Regional Diagnostic Disease Laboratory (WRDDL). Purposive sampling, also known as judgmental, selective or subjective sampling, is a type of non-probability.
sampling technique, which focuses on sampling techniques where the units that are investigated are based on the judgment of the researcher. Serum samples collected for other sero monitoring purposes were used in this general purpose study and represented a non-purposive sampling (Renukaradhya et al., 2002).
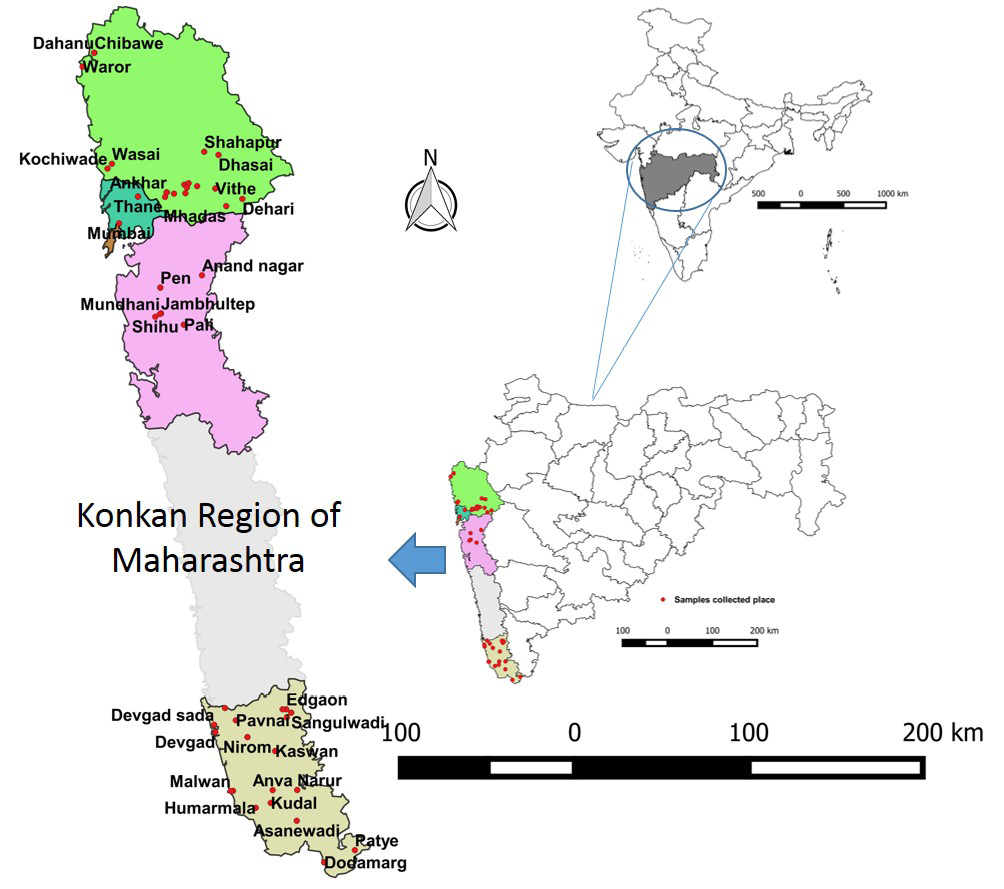
Figure 1: Map showing study area
Leptospira Media and Cultures
Ellinghausen McCullough Johnson and Harris (EMJH) liquid medium prepared as per the standard protocols using the EMJH base (BD Difco™, USA cat. # 279410) and Enrichment media (BD Difco™ USA, cat. # 648375) with 200 µg of 5-fluorouracil per ml of medium and was used for the propagation of reference leptospira serovars. These reference serovars were obtained from WHO National reference Laboratory, Regional Medical Research Centre (RMRC), Port Blair and are being maintained in biosafety laboratory at ICAR-NIVEDI for doing MAT (Table 2).
Table 1: Details of the serum samples from different areas in Konkan region of Maharashtra during survey in bovine population
|
District |
Taluk/area |
No. of samples screened for leptospira antibodies by MAT |
||||||
|
Cattle |
Buffaloes |
Bulls |
Bullocks |
Total |
Reacted |
Percent Positivity |
||
|
2011-2012 (Purposive Samples) |
||||||||
|
Raigad |
Pen |
3 |
24 |
3 |
- |
30 |
- |
- |
|
Pali |
4 |
- |
20 |
6 |
30 |
11 |
36.6 |
|
|
Sindhurdurg |
Kankavli |
7 |
- |
2 |
1 |
10 |
8 |
80.0 |
|
Kudal |
11 |
16 |
2 |
1 |
30 |
18 |
47.3 |
|
|
Devgad |
25 |
6 |
6 |
- |
37 |
3 |
8.10 |
|
|
Vaibhawadi |
7 |
3 |
30 |
- |
40 |
10 |
25.0 |
|
|
Malwan |
10 |
4 |
6 |
- |
20 |
3 |
15.0 |
|
|
Dodamarg |
9 |
10 |
1 |
- |
20 |
4 |
20.0 |
|
|
Thane |
Shahapur |
4 |
5 |
1 |
- |
10 |
4 |
40.0 |
|
Kalyan |
9 |
56 |
5 |
10 |
80 |
28 |
35.0 |
|
|
Murbad |
4 |
12 |
- |
14 |
30 |
21 |
70.0 |
|
|
Dahanu |
24 |
27 |
- |
39 |
90 |
33 |
36.6 |
|
|
Wasai |
11 |
8 |
- |
1 |
20 |
1 |
5.0 |
|
|
Sub Total |
128 |
171 |
76 |
72 |
447 |
144 |
32.2 |
|
|
2011-2012 (Non-purposive samples) |
||||||||
|
Raigad |
Raigad |
14 |
5 |
- |
- |
19 |
7 |
36.8 |
|
Sindhurg |
Sindhurg |
5 |
9 |
- |
- |
14 |
8 |
57.1 |
|
Sub Total |
19 |
14 |
- |
- |
33 |
15 |
45.5 |
|
|
2013-2014 (Purposive samples) |
||||||||
|
Sindhurg |
Sindhurg |
- |
2 |
1 |
- |
3 |
3 |
100 |
|
Raigad |
Raigad |
- |
1 |
1 |
9 |
11 |
11 |
100 |
|
Sub Total |
- |
3 |
2 |
9 |
14 |
14 |
100 |
|
|
2015-2016 (Purposive Samples) |
||||||||
|
Mumbai |
Mumbai Municipalities |
24 |
57 |
- |
- |
81 |
66 |
81.4 |
|
Sub Total |
24 |
57 |
- |
- |
81 |
66 |
81.4 |
|
|
Grand Total |
171 |
245 |
78 |
81 |
575 |
236 |
41.0 |
|
The serovars selected cause disease in animals or may be of use as sentinel serovars to measure the potential spread.
Microscopic Agglutination Test (MAT)
MAT was performed as described earlier (Faine, 1982; Shivraj et al., 2009; Balamurugan et al., 2014) on all the serum samples at 1:100 dilution using 18 reference serovars (Table 2). Each serum sample which gave positive reaction at 1:100 was further examined (random purposive samples received during 2012) by diluting it 2-fold starting from 1:100 to 1:3200 in order to determine the end point MAT antibody titres to know the prevalence of active infection. A MAT titre of 1: 100 or above is taken as positive reactor as per WHO/OIE manual for leptospirosis (WHO, 2011; OIE, 2013).
Statistical Analysis
The estimation of apparent prevalence with 95% confidence interval and statistical data analysis were carried out using Excel Microsoft 2013 and Statistical package for Social Sciences (SPSS) version 22 (IBM). Chi-square test was used as per standard statistical method (Snedecor et al., 1989) for testing the independence of seroprevalence of leptospira across age groups, between different livestock species and across the sample regions for statistical inference to determine significant difference.
RESULTS
The test results of serum samples are shown in Table 3. Out of total 575 sera tested, 236 samples at 1:100 dilution reacted in MAT representing the 41.04 % seroprevalence of leptospirosis in bovine population. Serum samples causing 50% of leptospires to agglutinate and/or lyse were considered positive reactor. Among coastal areas, high prevalence was observed in Mumbai (66/81=81.4%) followed by Thane (87/230=37.83%), Sindhudurg (56/174=32.1%) and Raigad (27/90=30.0%). Among the bovine, the
Table 2: Panel of leptospira reference serovars used in the microscopic agglutination test
|
Species |
Serovar |
Strain |
Serogroup |
|
L. interrogans |
Australis |
Ballico |
Australis |
|
L. interrogans |
Bankinang |
Bankinang 1 |
Autumnalis |
|
L. interrogans |
Canicola |
HondUtrech IV |
Canicola |
|
L. interrogans |
Hardjo |
Hardjoprajitno |
Sejroe |
|
L. interrogans |
Hebdomadis |
Hebdomadis |
Hebdomadis |
|
L. interrogans |
Pyrogenes |
Salinem |
Pyrogenes |
|
L. borgpetersenii |
Tarassovi |
Perepelicin |
Tarassovi |
|
L. interrogans |
Icterohaemorrhagiae |
RGA(ATCC443642) |
Icterohaemorrhagiae |
|
L. interrogans |
Pomona |
Pomona |
Pomona |
|
L. Santarosai |
Shermani |
1342 K |
Shermani |
|
L. inadai |
Kaup |
LT 64 - 68 |
Tarassovi |
|
L. kirschneri |
Grippotyphosa |
MoskvaV |
Grippotyphosa |
|
L. fainei |
Hurstbridge |
BUT 6 |
Hurstbridge |
|
L. borgpetersenii |
Javanica |
Poi |
Javanica |
|
L. noguchii |
Panama |
CZ 214 K |
Panama |
|
L. interrogan |
Djasiman |
Djasiman |
Djasiman |
|
L. interrogan |
Copenhageni |
M 20 |
Icterohaemorrhagiae |
|
L. interrogan |
Bataviae |
Swart |
Bataviae |
seroprevalence of 52.2 % in buffaloes, 34.5% in cattle, 32.1% in bullocks and 29.4% in bulls was observed. Further, the observed seroprevalence was more in calf (4/12=33.33%) than buffalo calf (14.3%= 3/21) though not statistically associated (χ2 = 0.50, p>0.10). The most prevalent serovars among the reactive samples were Australis and Hardjo. Calf serum showed exposure to Bankinang, Hebdomadis, Icterohaemorragiae, Hardjo, Australis serovars while buffalo calf exposed to Bankinang, Hebdomadis and Icterohaemorragiae. The predominant leptospira antibodies were determined by frequency of distribution of the serovars against Australis (23.61%) followed by Hardjo (19.44%), Hebdomadis (16.67%), Bankinang (15.28%), Icterohaemorrhagiae (14.58%), Kaup (9.03%), Tarassovi (7.64%), Pyrogenes and Javanica (6.94%), Canicola (6.25%), Pomona (2.78 %) and Hurstbridge (1.39%) for the purposive samples received during 2012. However, the prevalence was 45.45 % (15/33) was observed with 36.84% in cattle, 57.14% in buffaloes, when tested the non-purposive samples received during 2012. The predominant leptospira serogroup antibodies were against Australis (54.16%), Icterohaemorragiae (45.8%), Bataviae (45.8%), Sejroe (41.6%), Hurstbridge (41.6%), Tarassovi (37.5%), Grippotyphosa (37.5%), Djasiman (37.5%), Pyrogenes (33.3%), Pomona (33.3%), Panama (33.3%), Javanica (33.3%), Hebdomadis (29.16%), Shermani (29.16%), Autumnalis (25%), Tarassovi-(kaup serovar-25%) and Canicola (16%).
On district-wise analysis, it was observed that Autumnalis, Canicola, Pomona, Grippotyphosa, Djasiman, Panama serogroups antibodies in Sindhudurg; and Australis, Tarrasovi-Kaup, Hebdomadis, Hurstbridge, Grippotyphosa, Bataviae serogroups antibodies in Raigad were prevalent. In Mumbai the prevalence was 81.4% (66/81) with the predominant leptospira serogroup antibodies were against Shermani (41.3%), Tarassovi (33.33%), Australis (30.6%), Pyrogenes (28%), Pomona (25.3%). It is evident from the study that the seroprevalence of leptospira in cattle (χ2 = 4.97, p>0.05) is not associated of age, where as it is associated of age in buffaloes (χ2 = 9.98, p<0.01), bulls (χ2 = 21.26, p < 0.01), and bullocks (χ2 = 10.89, p<0.01) (Table 3). Further, the pooled analysis revealed that the seroprevalence across the species (χ2 = 22.7, p<0.01) and study regions (χ2 = 65.9, p<0.01) are associated.
DISCUSSION
Leptospirosis is widespread in diverse geo-climatic zones of the world, most significantly those countries having hot and humid temperature and is most common in tropical and subtropical areas with high rainfall. The disease is found mainly wherever animals and humans come into contact with the urine of infected animals through contaminated environments. The magnitude of the problem differs from different geographical locations. It has been known to occur in India since early parts of the 20th century. Many leptospirosis cases in animals and humans in India have been reported in the rainy season and seasonal outbreak has also been reported in coastal area especially in Maharashtra, Kerala, Gujarat, Odisha, Andhra Pradesh, etc. which were due to the heavy rainfall and flooding.
Studies of bovine leptospirosis in different parts of the world indicate that serovars responsible for reproductive
Table 3: Statistical analysis of results of samples screened for bovine leptospirosis
|
Cattle |
Buffaloes |
Bulls |
Bullocks |
|||||||||||||||||||||||
|
2011-2012 ( Purposive Samples) |
||||||||||||||||||||||||||
|
Age wise analysis |
||||||||||||||||||||||||||
|
Years |
T |
R |
P |
CI |
T |
R |
P |
CI |
T |
R |
P |
CI |
T |
R |
P |
CI |
||||||||||
|
< 3 |
32 |
5 |
15.6 |
3.1-28.2 |
32 |
7 |
21.9 |
7.6-36.2 |
4 |
2 |
50.0 |
1.0-99.0 |
8 |
- |
- |
- |
||||||||||
|
3 to 7 |
75 |
28 |
37.3 |
26.4-48.1 |
82 |
40 |
48.8 |
37.9-59.6 |
16 |
12 |
75.0 |
53.8-96.2 |
29 |
13 |
44.8 |
26.7-62.9 |
||||||||||
|
> 7 |
21 |
7 |
33.3 |
13.2-53.5 |
57 |
16 |
28.1 |
16.4-39.7 |
56 |
9 |
16.1 |
6.5-25.7 |
35 |
5 |
14.3 |
2.7-25.9 |
||||||||||
|
Total |
128 |
40 |
31.5 |
23.2-39.3 |
171 |
63 |
36.8 |
29.6-44.1 |
76 |
23 |
30.3 |
19.9-40.6 |
72 |
18 |
25.0 |
15.0-35.0 |
||||||||||
|
χ² Value |
4.971NS |
9.988*** |
21.256*** |
10.890*** |
||||||||||||||||||||||
|
2011-2012 (Non-purposive Samples) |
||||||||||||||||||||||||||
|
19 |
7 |
36.8 |
17.2-61.3 |
14 |
8 |
57.1 |
29.6-81.1 |
- |
- |
- |
- |
- |
- |
- |
- |
|||||||||||
|
2013-2014 ( Purposive Samples) |
||||||||||||||||||||||||||
|
- |
- |
- |
- |
3 |
3 |
100.0 |
100 |
2 |
- |
- |
- |
9 |
8 |
88.9 |
50.6-99.4 |
|||||||||||
|
2015-2016 (Purposive Samples) |
||||||||||||||||||||||||||
|
24 |
12 |
50.0 |
29.6-70.3 |
57 |
54 |
94.5 |
84.4-98.6 |
- |
- |
- |
- |
- |
- |
- |
- |
|||||||||||
|
Species wise analysis |
||||||||||||||||||||||||||
|
171 |
59 |
34.5 |
27.5-42.2 |
245 |
128 |
52.2 |
45.8-58.6 |
78 |
23 |
29.4 |
19.9-41.0 |
81 |
26 |
32.1 |
22.4-43.5 |
|||||||||||
|
χ² Value |
22.7*** |
|||||||||||||||||||||||||
|
District wise analysis-across species |
||||||||||||||||||||||||||
|
Raigad |
Sindhudurg |
Thane |
Mumbai |
|||||||||||||||||||||||
|
T |
R |
P |
CI |
T |
R |
P |
CI |
T |
R |
P |
CI |
T |
R |
P |
CI |
|||||||||||
|
90 |
27 |
30 |
21.0-40.7 |
174 |
56 |
32.1 |
25.4-39.7 |
230 |
87 |
37.8 |
31.6- 44.4 |
81 |
66 |
81.4 |
70.9-88.9 |
|||||||||||
|
Overall |
||||||||||||||||||||||||||
|
T |
R |
P |
CI |
|||||||||||||||||||||||
|
575 |
236 |
41.04 |
37.0-45.2 |
|||||||||||||||||||||||
|
χ² Value |
65.9*** |
|||||||||||||||||||||||||
T-Number of serum samples tested; R- positive reactor in microscopic agglutination test (MAT); CI- Confidence interval at 95% level; P-Percent sero positivity (%); *** represents significance (p< 0.01) level; NS: Not significant
losses vary depending on which serovars are locally endemic. The antibody may be due to any of the large number of different serovars. Since leptospira antibodies may be present for a considerable period of time after infection in the serum, so the seroreactivity may indicate the present or past exposure to leptospira antigens. The disease is common, in cattle in virtually all the states of India (Srivastava, 2008). Animal samples received from the coastal region, in which human death cases were reported are generally flood prone areas.
MAT is sensitive and quite specific, however, its use is rather limited in various laboratories owing to the problems related to the maintenance of leptospira sp. reference strains. Despite its universal use and numerous attempts by various investigators and organizations to standardize the test, it is difficult to obtain consistent results between laboratories. Since MAT is relatively serovar/serogroup specific test, it is a choice for seroepidemiologic studies. Hence, in this study, initially all the serum samples were tested at 1:100 dilution using 18 reference serovars. A MAT titre of ≥ 1:100 was taken as positive (WHO, 2011), which were considered to be from animals either previously exposed to the leptospira or in the carrier status or suffering from the disease. However, due to high endemicity of the disease in Maharashtra coastal region, while calculating the true reactor above the WHO/OIE recommenced titre of 1:100, the next MAT titre at ≥ 200, there were 114 samples reactors and were considered definite positive, which may represent the recently infected animals with leptospira. Because of absence of paired serum samples and clinical signs, the reactor of 1: 400 (n=69); 1:800 (n=46); 1:1600 (n=35) and 1:3200 (n=20) showed highly positive, which clearly indicate active recent infection conclusive of leptospirosis as described earlier (WHO, 2011; OIE, 2013).
Antibodies against different serovars have been reported from Karnataka (4.6%) (Srivastava et al., 1983), Andaman (24.2%); Tamil Nadu {51.4%- (87% in endemic farm also reported by (Natarajaseenivasan et al., 2011)} and Andhra Pradesh (10.5) {Cattle-12.2% and buffaloes (11.1%) mainly to Patoc, Icterohaemorrhagiae (Srivastava et al., 1983)} Maharashtra (7.3%) and Uttar Pradesh (4-8%) (Srivastava and Kumar, 2003), Gujarat (12.8%) (Patel et al., 2014) and Odisha (42.5%) (Balamurugan et al., 2013b) during different survey. Earlier investigations conducted since 1995 in India have revealed that the seroprevalence of leptospirosis in various states has been 5.4% in buffaloes, 7.5% in cattle, 12.5% in sheep, 14.6% in horses and 15.9% in dogs (Srivastava, 2008). However, the most of the other serovars reacted samples also showed multiple reaction with either of aforesaid major serovars, only few number of samples showed reaction with Pyrogenes (n=4), Canicola (n=6), Pomona (n=3) and Javanica (n=5) only. Thirty serum samples showed multiple serovar reactions in MAT, of which 24 samples have shown to reacted with Australis (n=7) and Hardjo (n=12). Only four sera showed highest antibody titers of 1:3200 against Hardjo and Australis serovars. At higher dilution at >1:400 of the positive reactive serum samples, reaction with serovars remain same with Australis and Hardjo, which may be considered as highly infective serovars in that particular geographical location.
As it is a well-known fact Hardjo serovar is the common one in cattle (Leonard et al., 2004), dairy cattle have a role as a natural host of serovars Hardjo, Pomona, and Grippotyphosa, while pigs may harbour Pomona, Tarassovi, and Bratislava, sheep may harbour serovars Hardjo and Pomona, and dogs may harbour serovar Canicola (Bolin, 2000). The present study also showed the similar results, in addition to this, the prevalence of serovars Australis and Hardjo in more than 20% case. However, the high antibody titres against these serovars indicate that the population is affected recently with these infective serovars. Infected animals are reported to shed the organism in their urine, aborted material discharge, body fluid and tissues. The possibility therefore exists that these apparently healthy seropositive animals may be shedding leptospira and they serve as source of infection to other animals and humans.
In earlier study, Srivastava and Kumar (2003) reported majority of the positive cases of human (n=265) were from Mumbai or Pune medical colleges with 43 (16.2%) of these showing positivity to various serovars while analysing the samples from various states and reported high level of the prevalence of leptospirosis in animals and humans warranting continuous investigations in order to suggest control strategies in the future. Similarly, presence of antibodies against Leptospira serovar Hardjo and Australis in bovine sera was earlier reported by Shivraj et al. (2009) which might be due to the cattle grazing in nearby areas of the water bodies. Further, it indicates the seroprevalence of leptospira in different study regions viz., Raigad, Sindhudurg, Thane, and Mumbai are not independent, which may be due to the same environmental, livestock rearing practices and other socioeconomic factors prevailing in these regions. When tested the non-purposive samples received during 2012 in MAT, which showed the predominant leptospira serogroup antibodies were against Hurstbridge, Tarrasovi-Kaup, Sejro, Javanica, Grippotyphosa, Autumnalis Shermani in Raigad district, and Hurstbridge, Autumnalis, Tarassovi, Icterohaemorragiae, Tarrasovi-Kaup, Javanica in Sindhudurg district with over all prevalence of 45.45%.
In general, Maharashtra state, having the contusive average rainfall and temperature which is very much ideal for the survival, maintenance and propagation of leptospirae. Epidemics may be associated with changes in human behaviour, animal or sewage contamination of water, changes in animal reservoir density, or follow natural disasters such as cyclones and floods. In a season record, 210 mm rainfall was recorded in a single day, where more than 100 domestic animals died and hundreds of houses were damaged. The total rainfall recorded on a single day in the Arvi district was 870.8 mm (http://nraa.gov.in). The samples were also received during the same period for testing and this proves that region close to the coastal area where the rainfall and flood rate are relatively high and cause for prevalence. Another reason is the crops that these regions grow are rice, pulses, millets etc. and have lots of paddy fields. Due to the agricultural fields and there is a raise in the population of the rat, which are the reservoir of leptospirosis, may play in the transmission of the disease to the humans. Further, there is a high use of fertilizers, which maintains the alkaline pH providing a suitable environment for the prevalence of leptospira. These coastal areas are also bordered with or passing through forest zone of Sahyadri ranges, hence the possibilities of the spread of leptospirosis from the wildlife especially rodents can also be considered. More of urban area in Thane district, where the water supply and sanitation are let down due to the growing population, which may be more contaminated with the Leptospira sp.
The major reactive serovars in these regions were Australis, Hardjo, Hebdomadis, Bankinang, Icterohaemorrhagiae, Kaup, Shermani, etc., The reactive Leptospira intermediate species serovars namely Kaup, Tarassovi, Hurstbridge, Shermani may of significance as the prevalence of intermediate species in India has been reported (Balamurugan et al., 2013a; Balamurugan et al., 2014). Hence, further systematic random screening of the samples will determine the exact prevalence of serovars, in this region, which will enable to select the required or prevalent limited panel of serovars representing their respective serogroups to be used for MAT without false negative in the diagnostic laboratory for providing early diagnosis of leptospirosis in humans as well as in animals and prompt treatment to be taken to reduce the extent of problem caused by the leptospira and the economic losses associated with it.
In conclusion, significant prevalence of leptospirosis in the coastal region of Maharashtra proves the endemic zone for leptospirosis as indicated by the high seroprevalence in bovine species on screening by MAT and also earlier reported study. This study supports that bovines may have a role in maintaining Australis, Hardjo, Hebdomadis, Bankinang, Icterohaemorrhagiae, Kaup, Shermani, etc., serovars apart from being a well-known reservoir for Hardjo serovar. These major serovars in these region can be included in the test panel of serovars to be used for MAT in both human and animal disease diagnostic laboratories for providing early and proper diagnosis of leptospirosis in order to undertake prompt treatment. The high seroprevalence of leptospira serovars in apparently healthy bovine indicates the host-adapted serovars involves and produce subclinical infection in animals and serving as chronic carriers and persistent shedders through their urine, body fluid or tissue (organism in the environment), which may be a potential zoonotic risk to farmers, milkers, and other domestic species. This study shows that as the leptospirosis prevalence as a zoonotic disease, the title as the re-emerging zoonotic disease holds well and awareness about the disease has to be implemented in a proper way. The study also determines the need for continuous monitoring of the disease burden in animals and humans in close proximity to each other to combat zoonotic diseases.
ACKNOWLEDGEMENTS
Authors wish to thank Indian Council of Agricultural Research (ICAR), New Delhi, India, for encouragement and financial support. The authors also thank the Joint Commissioner of Animal Husbandry, Disease Investigation Section (WRDDL), Aundh, Pune, Maharashtra for periodically sending the samples to the ICAR-NIVEDI for diagnosis. The authors also thank Dr. P. Vijayachari, Director, Regional Medical Research Centre (ICMR), Port Blair, for providing the leptospira reference cultures and also for his constant support and encouragement in research. The authors also thank the staff of ICAR-NIVEDI for their constant support and timely help.
CONFLICT OF INTEREST
No conflicts of interests are declared by authors for the contents in the manuscript.
AUTHORS CONTRIBUTION
V. Balamurugan designed and carried out the experiment, interpreted the data and wrote the draft of manuscript. S.R.A. Thirumalesh, S. Veena, A. Alamuri, and R. Sridevi carried out the experiment. G. Govindaraj and M. Nagalingam, performed the statistics, interpreted the data and provided support and edited manuscript. D. Hemadri, M.R. Gajendragad and H. Rahman made arrangement of the samples and provided guidance and support to carry out the experiments.
REFERENCES





