Advances in Animal and Veterinary Sciences
Research Article
Ocular Dermoids in Crossbred Indian Cattle: A Comparative Evaluation of Four Year (2012 – 2015) Study Results and Literature Reviews
Sherin B Sarangom, Kiranjeet Singh*, Aswathy Gopinathan, P Sangeetha, Nitish Kallianpur, S Shivaraju, K Praveen, Deepti Sharma, Priya Singh
Division of Surgery, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, 243 122, India.
Abstract | An exclusive literature on ocular dermoids in crossbred Indian cattle is currently unavailable except for sporadic reports. Medical records of past four years (2012-2015) were investigated for evaluation of ocular dermoids in crossbred Indian cattle. The overall incidence of ocular dermoids was found to be 0.345% (5/1451). All the animals were less than five months of age. Based on anatomical location, four types of ocular dermoid were noticed either alone or in combination, both unilaterally and/or bilaterally. Unilateral presentation was common and the ventrolateral corneoconjunctival dermoid predominated by type. Bilateral coexistence of both ventrolateral corneoconjunctival dermoid and medial canthus dermoid in a single eye was noticed in a calf and is believed to be the first report of this kind. Histopathological evaluation of the ocular choristomas showed hair shafts and bulbs in addition to sebaceous and sweat glands. Postoperative evaluation showed adequate response to the surgical treatment adopted without any subsequent recurrence. Following surgical excision, a calf with bilateral corneoconjunctival dermoid repaired by superficial keratectomy and conjunctival flap developed iris prolapse, iridocyclitis and phthisis bulbi and subsequently underwent unilateral enucleation of eyeball. The acquired data was compared and evaluated with literature reviews.
Keywords | Ocular dermoid, Indian cattle calves, Superficial keratectomy
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 22, 2015; Revised | November 16, 2015; Accepted | November 18, 2015; Published | December 11, 2015
*Correspondence | Kiranjeet Singh, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India; Email: ksuppli@yahoo.co.in
Citation | Sarangom SB, Singh K, Gopinathan A, Sangeetha P, Kallianpur N, Shivaraju S, Praveen K, Sharma D, Singh P (2016). Ocular dermoids in crossbred Indian cattle: A comparative evaluation of four year (2012 – 2015) study results and literature reviews. Adv. Anim. Vet. Sci. 4(1): 46-52.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.1.46.52
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Sarangom et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ocular dermoids are choristomatous defects characterized by the overgrowth of normal benign tissue that arise from the ocular region congenitally (Shields et al., 1986). Usually, they are located in limbus, cornea, conjunctiva, corneoconjunctiva (Lawson, 1975) but also have been noticed in membrana nictitans and eyelid (Roh et al., 2014). Dermoids have been reported frequently in dogs (Barnett et al., 2002; Bodh et al., 2015) and cattle calves (Al-Badrany et al., 1989; Yeruham et al., 2002; Roh et al., 2014), less in cats (Glaze, 2005) and rarely in other domestic animals like horse (Joyce et al., 1990; Greenberg et al., 2012), donkey (Misk et al., 1994), buffalo (Rezaei et al., 2007), goat (Pimentel et al., 2007), sheep (Bukar et al., 2008), camel (Moore et al., 1999) and pig (Brightman et al., 1985), wild animals like white tailed deer (LaDouceur et al., 2012), red deer (Gelmetti et al., 2010), lion (Robinson and Benirschke, 1981) and wildebeest (Weber and vanHoven, 1990), birds like parrot (Leber and Burge, 1999) and goose (Busch, 1985), laboratory animals like rat (Nichols and Yanoff, 1969), rabbit (Styer et al., 2005), guinea pig (Otto et al., 1991; Wappler et al., 2002) and even in humans (Pirouzian, 2013). Among cattle, the condition has been evaluated and reported exclusively in native Korean cattle (Roh et al., 2014), Hereford cattle (Barkyoumb and Leipold, 1984; Brudenall et al., 2008) and Israeli-Holstein breed (Yeruham et al., 2002). Although sporadic occurrence of the condition has been reported in crossbred Indian cattle calves (Bhatt et al., 1964; Misra and Angelo, 1979; Bose et al., 1982; Purohit et al., 1987; Sarma and Sarma, 1989; Simon et al., 2010; Tanwar and Gahlot, 2012), a detailed investigation on the incidence of ocular dermoid in them and a long term study is lacking. Hence, the study was undertaken to determine the incidence of ocular dermoids in crossbred Indian cattle and to evaluate and develop a preliminary database based on various observations made in them on the light of available literatures.
Material and Methods
Medical records of crossbred Indian cattle presented to the Referral Veterinary Polyclinics, Indian Veterinary Research Institute, Izatnagar, India from January 2012 to July 2015 were collected. The records of cattle presented with ocular dermoids were investigated in detail and the incidence, the age and sex of the animals were determined. The type and nature of ocular dermoids were assessed based on ophthalmic examination. The extend and invasiveness of the lesion, accompanying ocular malformation, the type and effectiveness of the treatment adopted, the complications observed following treatment and the recurrence of the condition were also evaluated. Gross and histopathological evaluation of the excised masses was performed. The results of the study were compared with the available literatures on ocular dermoid in cattle, particularly in crossbred Indian cattle.
Results
A total of 1451 crossbred Indian cattle (920 adults, >6 months and 531 calves, <6 months) were presented during the period of study with various ailments. Out of the total hospital population, 967 animals (597 adults and 370 calves) were presented with various surgical conditions. Among them, 41 animals (27 calves and 14 adults) were found to have various ocular affections. Ocular dermoids were diagnosed in five animals (Table 1). All the animals were less than six months of age. This accounted 18.52% of the calves and 12.20% of the total cattle with ocular affections, respectively. When the animals presented with different surgical conditions are considered, the animals with ocular dermoids constituted 1.351% and 0.517% of the calves and total cattle population, respectively. An incidence of ocular dermoids was estimated to be 0.942% among cattle calves and 0.345% among total cattle based on hospital population. An age based comparative graphical representation of data on ocular dermoids to other conditions and hospital population is depicted in Figure 1.
Of the five calves diagnosed to have ocular dermoids, three were females and two were males with a mean age of 2.4 ± 0.51 months ranging from 1 to 4 months and weighing 30-60 kg. Unilateral dermoid was observed in four calves with the left eye being the most commonly affected in three calves and right eye in one calf. Obviously, one calf had bilateral dermoid. There was marked variations in extend of dermoids that ranged from a small growth to moderate size with accompanying mild ocular malformations. In animals with dermoids of moderate size, vision was partially impaired but preserved. The dermoid affected only the superficial corneal layers in all the calves with corneal involvement. According to the Barkyoumb and Leipold (1984) nomenclature, four type of dermoids were noticed which included ventrolateral corneoconjunctival dermoid (three calves), superotemporal corneal limbal dermoid, medial canthal dermoid and membrana nictitans dermoid (one calf each) with mild disfigurement of ocular structures. The calf with bilateral dermoid had both corneoconjunctival dermoid and medial canthal dermoid on the same eye (Figure 2). The initial noticeable sign to the owner in all the cases was a small irregular mass with a tuft of hair in the eye. Detailed history revealed that the mass was present since birth but had gradually increased in size till the day of presentation. The common clinical symptom observed was the presence of moderate to severe epiphora from the affected eyes. Other clinical signs included corneal opacity, mild blepharospasm, conjunctivitis and distortion of pupil. There was mild to moderate disfigurement of cornea with varying degrees of encroachment of dermoid tissue on to the
Table 1: The crossbred Indian cattle calves presented with ocular dermoids in our study
|
Sl. No. |
Breed |
Age (Months) |
Sex |
Eye affected (Right/Left) |
Unilateral/ Bilateral |
Type of dermoid |
Ocular malformation |
Treatment performed |
Complication/ Recurrence if any |
|
1 |
CB |
2 |
M |
Left |
Unilateral |
Corneoconjunctival |
Distorted pupil |
Superficial keratectomy Conjunctival flap |
- |
|
2 |
CB |
1 |
F |
Left |
Unilateral |
Corneoconjunctival |
Conjunctivitis |
Superficial keratectomy Conjunctival flap |
Corneal scarring |
|
3 |
CB |
2 |
M |
Right |
Unilateral |
Membrana nictitans |
Corneal opacity |
Surgical excision |
- |
|
4 |
CB |
4 |
F |
Left |
Unilateral |
Corneolimbal |
- |
Superficial keratectomy Conjunctival flap |
Corneal scarring |
|
5 |
CB |
3 |
F |
Both |
Bilateral |
Corneoconjunctival, Medial canthal |
Conjunctivitis |
Superficial keratectomy Conjunctival flap |
Iris prolapse, Phthisis bulbi, Iridocyclitis |
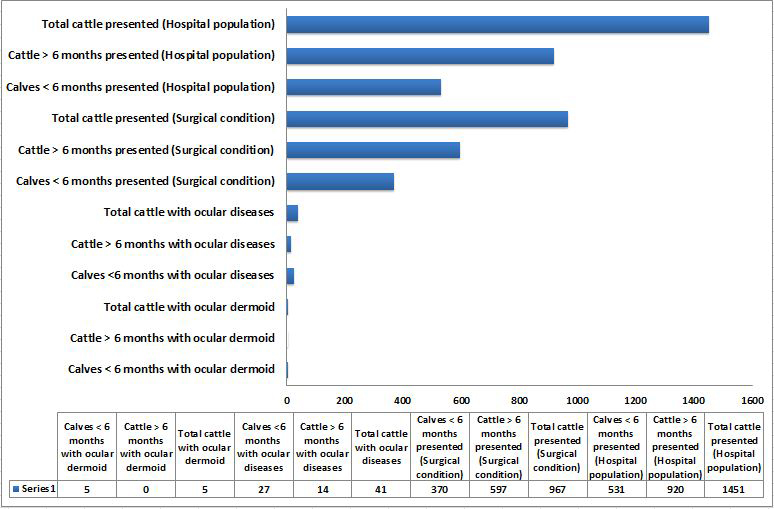
Figure 1: Graphical representation of hospital data on number of calves less than 6 months of age and cattle above 6 months of age presented with ocular dermoids, ocular diseases and other conditions
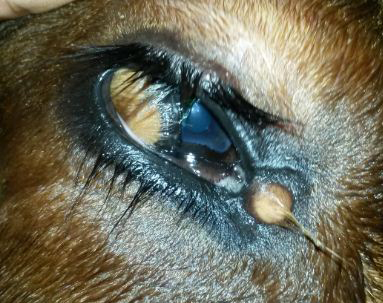
Figure 2: The ventrolateral corneoconjunctival dermoid on the right eye of a calf with haired corneal tissue merging with the hairless tissue of conjunctiva
A minute dermoid was noticed in the medial canthus also. The dermoid was bilateral and symmetrical visual axis. All the calves were in good body condition with normal physiological and haemato-biochemical values.
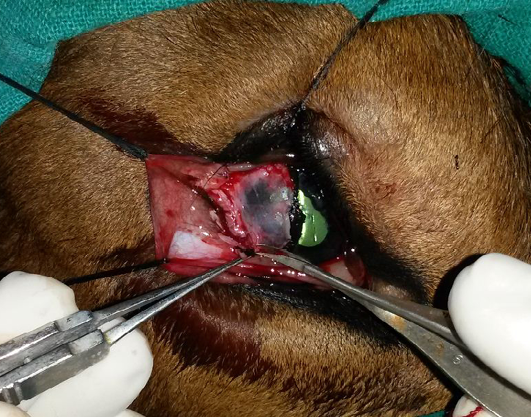
Figure 3: Excision of the conjunctival dermoid and superficial kerectotomy performed for the removal of the corneoconjunctival dermoid
Surgical repair was attempted in all the calves except for medial canthus dermoid under a combination of auriculopalpebral and Peterson nerve block, 8-12ml of 2% lignocaine hydrochloride (Xylocaine 2%, AstraZeneca Pharma India Limited, Dundigal post, India) and topical anaesthesia using 4% lignocaine hydrochloride (Xylocaine 4%, AstraZeneca Pharma India Limited, Dundigal post, India). Surgical repair included excision of membrana nictitans dermoid and superficial keratectomy followed by conjunctival flap for corneolimbal and corneoconjunctival dermoids (Figure 3 and 4). Topical ciprofloxacin eye drops (Ciplox, Cipla Ltd., Mumbai, India) were instilled thrice daily (TID) and was started three days prior to surgery. Post-operatively, 1% atropine sulphate eye drops (Bellpinoatrin 1%, Bell Pharma Pvt. Ltd., Mumbai, India) instilled twice daily (BID) topically for 5 days, ciprofloxacin eye drops TID topically for 7 days in addition to ceftriaxone (Intacef, Intas pharmaceuticals, Ahmadabad, India) @ 10 mg/kg BW IV, BID for 7 days and meloxicam (Mel onex, Intas pharmaceuticals, Ahmadabad, India) @ 0.2mg/
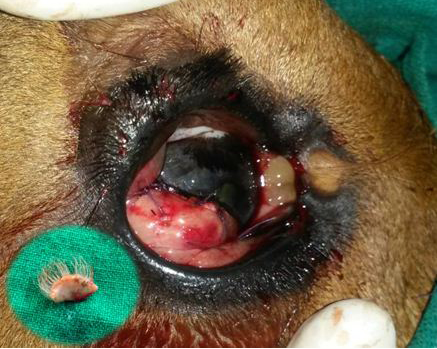
Figure 4: Postoperative photograph of the conjunctival flap applied on to the cornea for augmenting healing of cornea following superficial keratectomy
The minute dermoid on the medial canthus was left unoperated. The inset shows the excised corneoconjunctival dermoid.
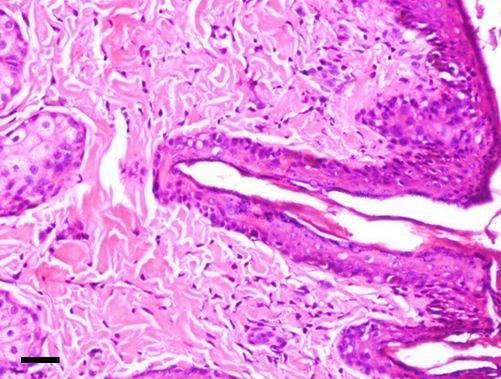
The dermoid showed stratified squamous keratinized epithelium above a thick collagenous stroma with hair follicles and bulbs in addition to apocrine gland [H&E; bar = 100 μm]
kg BW IM, once daily (OD) for 3 days were administered. Following surgical repair, all the calves showed adequate response to the surgical treatment adopted without any subsequent recurrence. Complication was noticed in a calf with bilateral ocular dermoid that underwent unilateral enucleation of eyeball subsequent to the development of iris prolapse, iridocyclitis and phthisis bulbi following a traumatic incident during the postoperative period. Slight corneal scarring was noticed in two calves with corneoconjunctival dermoid following surgical repair.
Grossly, the excised dermoid appeared as moderately delineated and non-capsulated mass. Except the conjunctival tissue, all the masses had hairs overgrowing from it. On histopathological examination, the corneal masses were composed of stratisfied squamous keratinized epithelium layered above a stroma of collagenous nature with numerous well developed hair follicles. The corneal tissue merged with conjunctival mass embedded with glandular tissue. The epidermis and dermis of corneoconjunctival masses exhibited well developed hair shafts and bulbs in addition to apocrine and sebaceous glands (Figure 5). The masses appeared similar in histopathology of bilateral dermoids.
Discussion
The incidence of ocular dermoid has been documented to be less in many breeds across the world (Greene et al., 1973; Yeruham et al., 2002). In our study, the incidence of ocular dermoid was found to be rare in crossbred Indian cattle also and accounted 0.345%. Previously, the incidence of ocular dermoids has been estimated to be between 0.0025% (Yeruham et al., 2002) and 0.4% (Greene et al., 1973). Herewith, it should be assumed that crossbred Indian cattle calves also does not have any apparent breed predisposition that has been noticed by Barkyoumb and Leipold (1984) in Hereford cattle. The affected calves belonged to different regions and do not had any herd based relationships. A heritable autosomal recessive and polygenic trait have been speculated in Hereford cattle. Similar description of familial inheritance and breed predisposition for ocular dermoids has been described in Burmese and Birman cats (Koch, 1979; Hendy-Ibbs, 1985), Dalmatian, German Shepherd, Doberman and Saint Bernard dogs (Barnett et al., 2002), Quarter-horses (Joyce et al., 1990) and even guinea pigs (Otto et al., 1991). The available literature on crossbred Indian cattle over past 50 years also does not emphasize any breed specific inheritance of this condition (Bhatt et al., 1964; Misra and Angelo, 1979; Purohit et al., 1987; Simon et al., 2010; Pandey et al., 2011; Tanwar and Gahlot, 2012).
All the animals in our study were found to be less than 4 months of age although ocular dermoids have been reported in cattle up to 2 years of age (Roh et al., 2014). Among crossbred Indian cattle, ocular dermoid have been reported up to 7 months of age (Mudasir et al., 2012). Also, the age of calves on the day of presentation was accounted rather than the day on which the initial signs were observed by the owner that was considerably less. No clear cut sex based predisposition has been noticed. The available case reports on crossbred Indian cattle calves also does not indicate any sex based predisposition for ocular dermoids although an increased incidence of crossbred Indian female cattle calves affected with ocular dermoid have been reported by Simon et al. (2010) and Tanwar and Gahlot (2012). Of the five cases in our study, the predominant dermoid was ventrolateral corneoconjunctival dermoid. An increased incidence of ventrolateral corneoconjunctival was also reported by Barkyoumb and Leipold (1984) and Yeruham et al. (2002) compared to the inferonasal corneoconjunctival dermoid reported by Brudenall et al. (2008) and Roh et al. (2014). However, both ventrolateral and inferonasal corneoconjunctival dermoids have been described in crossbred Indian cattle (Bhatt et al., 1964; Bose et al., 1982; Bose and Karimullah, 1984; Purohit et al., 1987; Ansari and Moulvi, 2013). The located sites of ocular dermoids were similar to that reported by Roh et al. (2014) in Korean cattle, Barkyoumb and Leipold (1984) in Hereford cattle and Yeruham et al. (2002) in Israeli-Holstein cattle. But Barkyoumb and Leipold (1984) located most of the dermoids in the ventrolateral limbus followed by third eyelid, canthus, eyelid and conjunctiva whilst the corneoconjunctival dermoid predominated in Indian cattle. The canthal dermoid was medial as observed by them but had bilateral occurrence in conjunction with corneoconjunctival dermoid.
Unilateral occurrence was observed to be common compared to the bilateral involvement as observed by Yeruham et al. (2002). Although the unilateral occurrence is common, bilateral dermoids have also been reported in cattle (Al-Badrany et al., 1989; Brudenall et al., 2008; Kilic et al., 2012). Breed specific increased incidence of bilateral dermoids was observed by Barkyoumb and Leipold (1984) in Hereford cattle. Among Indian cattle, bilateral ocular dermoids were reported previously by Bhatt et al. (1964), Bose et al. (1982), Bose and Karimullah, (1984), Purohit et al. (1987), Pratap et al. (2005), Simon et al. (2010) and Nagar et al. (2015). According to them, the bilateral presentation of either inferonasal corneoconjunctival dermoid or medial canthal dermoid was frequent although ventrolateral corneoconjunctival dermoid has also been reported (Nagar et al., 2015). But, the coexistence of both corneoconjunctival dermoid and medial canthal dermoid in the same eye presented bilaterally in a calf noticed in our study was unique and is believed to be the first report of its kind although occurrence of any similar or different type of single dermoid bilaterally have been observed. Also, both the dermoids were symmetrical in appearance bilaterally. Bilaterally symmetrical single type of dermoid on each eye was reported by Brudenall et al. (2008). But, bilateral symmetrical presentation of two different types of dermoid in each eye is not documented elsewhere. Location of dermoids elsewhere other than ocular structures (Barkyoumb and Leipold, 1984; Roh et al., 2015), ocular abnormalities and anomalies (Bukar et al., 2008), concurrent choristomatous defects (Barkyoumb and Leipold, 1984; Brudenall et al., 2008; Kilic et al., 2012) and other congenital malformation and anomalies (Barkyoumb and Leipold, 1984; Yeruham et al., 2002; Roh et al., 2014) were not noticed in our study. The only available report on concurrent abnormality in an Indian cattle calf was the presence of a tumor like growth at the entrance of left nasal passage in an Indian Rathi calf (Bhatt et al., 1964).
Corneal dermoids were removed successfully by superficial keratectomy while conjunctival dermoids by conjunctivectomy or through a simple surgical excision of dermoid tissue employed for membrana nictitans dermoid. Early complete excision is warranted else the long hairs arising from the surface of tissue may cause conjunctival and corneal irritation leading to chronic epiphora, conjunctivitis and keratitis (Greene et al., 1973; Greenberg et al., 2012; Glaze, 2005; Pandey et al., 2011) and pigmentary keratitis with subsequent visual impairment in prolonged cases (Kilic et al., 2012). The medial canthus dermoid was not operated as it inflicted no hindrance to the normal ocular function and the dermoid was too small and dormant. Other than corneal scaring, the complication observed in a calf that underwent unilateral enucleation of eyeball during the postoperative period was due to self-inflicted trauma that was unusual. Postsurgical complications were uncommon with ocular dermoids except for corneal scaring that was reported by Al-Badrany et al. (1989).
The pathogenesis of ocular dermoids is still unclear. An abnormal differentiation of surface epithelium as a result of metaplasia of the neural ectodermal mesenchyme has been proposed by Cook (2007). A variety of possible causes have been enlisted by Barkyoumb and Leipold (1984), Barnett et al. (2002) and Pimentel et al. (2007). The dermoids had ectodermal elements like keratinized epithelium, hairs, sebaceous and apocrine glands in addition to mesenchymal elements like fibrous tissue, fat and cartilage combined in different proportions. The gross appearance and components of dermoids in our study were found similar to that reported by Brudenall et al. (2008), Kilic et al. (2012) and Roh et al. (2014). Even though our investigation could present data related to the overall incidence, clinical presentation and ocular relationships and characteristics, the etiopathogenesis could not be determined. Also, the inheritance of this condition in native crossbred calves could not be assessed as the data pertaining to a defined herd was not available. However, the available literatures have not duly demonstrated the inheritance of ocular dermoids in crossbred Indian cattle.
Genetic predisposition of ocular dermoids is well established in certain breeds of canines, felines and bovines. The present study and available literatures on ocular dermoid in Indian cattle calves suggests it as a rare entity in newborn. Also, the occurrence of any concurrent congenital anomalies was not noticed in any crossbred Indian cattle calves similar to that described in Hereford cattle. A herd based study in a breeding population is still warranted to reveal any inheritance or breed predisposition in addition to study the exact etiology behind the development of this condition.
Acknowledgement
The authors are thankful to the Director, ICAR-Indian Veterinary Research Institute, Bareilly, U.P., India for providing necessary facilities for the study.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors’ Contribution
All the authors contributed equally.
References