Advances in Animal and Veterinary Sciences
Research Article
Estimation of Toxic Hazards Caused by Methanolic Extract of Euphorbia peplus in Male Albino Rats
Gihan Gamal Moustafa, Amany Tharwat Mohammed*, Ali Heider Abo Hadeed, Fatma Hassan Kandil, El Kelsh Moustafa El Kelsh
Forensic Medicine and Toxicology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt.
Abstract | Euphorbiaceae family presents some pharmacological activities that its latex can be used widely in the traditional medicine as a germicide, antiparasitic and in asthma and cough healing. Although the huge amount of literatures that discuss the beneficial and therapeutic uses of Euphorbia, attention had hardly paid to the adverse effects of the latex of this family. Euphorbia peplus (petty spurge or milkweed) is a species of Euphrbia that native to North Africa where it typically grows in the cultivated lands and gardens. This study was aimed to explore the toxic hazards of Euphorbia peplus latex in male albino rats using different assays including estimation of testosterone and leutinizing (LH) hormones , liver enzymes, total protein and the antioxidant enzymes superoxide dismutase and glutathione reductase in serum samples. Also, studying the effect of plant latex on testicular and liver tissue DNA fragmentation. The plant was stored in 95% methanol at room temperature for extraction of the plant. The oral administration of 500mg/kg b.wt of plant latex twice weekly for 65 days revealed a significant reduction in weight of testis and seminal vesicle with a significant decrease in sperm cell concentration and sperm motility as compared with control group. Meanwhile, the percentage of sperm abnormalities was significantly increased. Serum levels of testosterone and LH hormones were significantly decreased. The antioxidant enzymes superoxide dismutase, glutathione reductase and catalase showed a significant decrease. The latex provoked an obvious DNA damage and fragmentation in both testicular and hepatic tissues after oral treatment with euphorbia peplus latex as compared to control group.
Keywords | Euphorbia peplus, Male fertility, Rat, DNA fragmentation, DNA damage
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 07, 2015; Revised | November 11, 2015; Accepted | November 12, 2015; Published | November 29, 2015
*Correspondence | Amany Tharwat Mohammed, Zagazig University, Zagazig, Egypt; Email: amany_tharwat@yahoo.com
Citation | Moustafa GG, Mohammed AT, Abo Hadeed AH, Kandil FH, El Kelsh EKM (2016). Estimation of toxic hazards caused by methanolic extract of Euphorbia peplus in male albino rats. Adv. Anim. Vet. Sci. 4(1): 5-11.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.1.5.11
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Moustafa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Family Euphorbiaceae comprises over 7500 species within 283 genera. The genus Euphorbia consists of more than 1600 species growing nearly in all types of climates throughout the world (Boulos, 1980). Herbal medicines was found to be an important source for the developing countries in controling several complications and troubles of reluctance and side effects of the existing antimicrobial agents. The World Health Organization (WHO) concluded that 80% of the the population of developing countries nearly use traditional medicines. This means approximately 3300 million people use medicinal plants on a regular basis. Medicinal plants used in traditional medicine should therefore be studied for safety and efficacy (Eloff, 1998). Euphorbia peplus L. is primarily domestic to Europe and North Africa (Zhi-Qin et al., 2010). Its milky sap has long been used as a treatment for skin cancers especially non-melanoma skin cancer (NMSC) and the active ingredients have been established as diterpene esters (Ramsay et al., 2011). Adedapo et al. (2003) explored that the aqueous extract of E. hirta caused diverse amount of testicular disintegration as well as decrease in the mean seminiferous tubular diameter in the treated rats. It consequently showed that the aqueous extract of E. hirta has a propable harmfull effects on the testes and accessory organs of rats. The authors revealed that great wariness should be taken in the use of these plants for medicinal applications. Oyeyemi et al. (2009) found that oral dosing of adult sexually matured West African Dwarf rams with Euphorbia hirta extract for 14 days showed reduction of sperm motility and live- dead ratio which elucidated that the fertilization ability and activity of spermatozoa were influenced. Sharma et al. (2007) declared that the aqueous extract of E. hirta exhibited an antioxidant effect and a free radical scavenging capacity in various in vitro models like total antioxidant and total ferric reducing power determination. Ozlem and Tulay (2012) reported that E. platyphyllos extracts at dose level (200g) showed a significant cytotoxic effect and provoked DNA fragmentation. Hamsa et al. (2013) found that the latex of Euphorbia antiquoram was able to induce apoptosis with a maximal DNA damage in spleen cells, which are non-cancerous and untransformed cells. Although the huge amount of literatures that discuss the beneficial and therapeutic uses of Euphorbia spp., little has paid any attention to the adverse effects of different plants of genus Euphorbia especially Euphorbia peplus on semen picture and DNA damage. Therefore, this study was designated to investigate the toxic impacts of Euphorbia peplus.
Material and methods
Plant (Euphorbia peplus)
The plant was freshly collected from their natural habitats, Abo-Hammad, Sharkia, Egypt. Whole plant (6 kg) were used in all extraction. Plant were chopped in small pieces and stored in methanol (95%) at room temperature directly after collection.
The dose used in our study was 500mg/kg b.wt of Euphorbia extract was administrated orally by stomach gavage twice weekly, for 65 days according to Al-Okbi et al. (2002).
Experimental Animals
Thirty mature male albino rats weighting 180-200 gm were obtained from the Laboratory Animals unit, Faculty of Veterinary Medicine, Zagazig University, were kept under hygienic measures, housed in metal cages. Rats were randomly separated into three equal groups (10 animals/ each group). Room temperature and humidity were kept at 23.0 ± 2.0°C and 50.0 ± 5.0% respectively during the study. The rats were accommodated to laboratory conditions fed ad-libitum and fresh water were available during the experimental period for two weeks before the experiment is going on, the light cycle 12/12 hrs dark/light. Drinkers and feeding troughs were daily cleaned.
Extraction of the Crude Base of Euphorbia peplus
Whole plants of Euphorboa peplus (6kg) were extracted with MeOH (1.8 : 1) at room temperature. The crude extract was concentrated in vacuum and splitted between CH2CL2 and filtered H2O. Evaporation of the organic phase gave a residue (47 g), which was chromatographed over a polyamide column, with mixtures of H2O/MeOH (3:2 and 1:4) as eluents Hohmann et al. (1991).
Experimental Design
Mature male rats were kept into two main groups that orally administered the solvent dimethyl sulfoxide (DMSO) and Euphorbia extract dissolved in DMSO in a dose of 500 mg /kg b.wt. two times per week for 65 days. The third group was kept as negative control group that received no treatment.
Sample Collection and Preparation
After slaughtering, blood samples were collected for separation of the serum which kept at -20°C till used in examination of testosterone hormone, luteinizing hormone, superoxide dismutase and glutathione reductase enzymes. Samples from testis and liver of all rats were taken and kept at-20°C for DNA fragmentation analysis. Another tissue samples from testis, prostate, seminal vesicles and liver were taken and fixed in 10% neutral buffered formalin till histopathological inspection.
Semen Picture Analysis
The cauda epididymis of one testis was removed and received in a sterile Petri dish containing warm normal saline, and then it was macerated by sterilized scissors to get the epididymal contents in a suspension that was handled as the semen (Hafez, 1970). Sperm motility assay was performed after the method represented by Slott et al. (1991). Sperm cell concentration per ml of semen was performed according to the method of Robb et al. (1987) and sperm abnormalities assay was determined using the method of Filler (1993).
Estimation of Biochemical Parameters
Testosterone levels were measured according to Wheeler (1995). The serum levels of LH were determined according to Tietz (1995).
Estimation of Serum Antioxidant Enzymes Level
Superoxide dismutase activity was assayed according to Niskikimi et al. (1972). Glutathione reductase activity was determined according to Beutler (1975). Catalase was measured according to Sinha (1972).
DNA Fragmentation Analysis of Liver and Testis
DNA damage was determined by DNA fragmentation assay according to the protocol which could be summarized as following: 5 X 106 target cells were labelled with 50µL of 3H-TdR (1 mCi/mL) overnight in 10 ml of media. The next day, the cells were washed 3X with 10ml of PBS and incubated in 10ml of media to chase out unincorporated cytoplasmic 3H-TdR. After incubating for 2 hrs, the cells were washed 3X with PBS and then used in lytic assay under the same conditions as the 51Cr release assay in 96 v-well plates. At the end of the assay, each well was treated with 20µL of 1.0% Triton-X on ice for 5 minutes, followed by centrifugation at 1500g in a Beckman T-J6 rotor for 15 minutes. 100µL of the supernatant were harvested from each well and counted in a scintillation counter. Total count was obtained by resuspending the cells prior to harvesting, and adding 0.1% SDS to solublilize the cells. The % 3H released was calculated with an equation analogous to that for %51Cr released Sellins and Cohen (1987).
Histopathological Investigations
Specimens from testis, seminal vesicle and protstate of both control and treated animals were fixed in10% neutral buffered formalin were processed for routine histopathological examination according to Bancroft et al. (1996).
Statistical Analysis
The obtained data were analysed and graphically represented using the statistical package for social science (SPSS, 8.0 software, 1997) for obtaining mean data and standard error. The data were analysed using one way ANOVA to determine the statistical significance of differences among animals groups SAS (1996).
Results
Weight of Reproductive Organs
After 65 days of treatment with crude extract of Euphorbia peplus (500mg/kg. B.wt.) twice weekly in mature male rats, there was a significant decrease in the weights of testis and seminal vesicle when compared with control one (Table 1).
Table 1: Effect of crude extract of Euphorbia peplus at dose level of 500mg/kg b.wt for 65 days on weight of reproductive organs of mature male albino rats
|
Organs |
||
|
Groups |
Testis |
Seminal vesicles |
|
Treated |
0.82+0.06b |
0.71+0.03b |
|
DMSO |
1.12+0.11ab |
0.90+0.02a |
|
Control |
1.21±0.10a |
0.87+0.02a |
Means carrying different superscripts within the same column are significantly different at (P<0.05) based on (least significant difference) LSD.
Effect on Semen Picture in Mature Rat
The obtained results detected that there was a significant reduction of sperm cell concentration compared with control one also there was a significant reduction of sperm motility. Meanwhile, there was significant elevation in proportion of sperm abnormalities compared together with control group (Table 2 and Figure 1).
Table 2: Effect of crude extract of Euphorbia peplus at dose level of 500mg/kg.B.wt for 65 days in mature male albino rats on the sperm picture (Means ± SE)
|
Parameters |
|||
|
Groups |
Sperm cell motility % |
Sperm cell concentration x 106/mm3 |
Sperm abnormalities (%) |
|
Treated |
66.67±1.67a |
17.67±4.70a |
31.67±6.49a |
|
DMSO |
80.00±2.89b |
31.88±4.91a |
10.00±1.15b |
|
Control |
83.33±3.33b |
32.33±3.93a |
12.33±.88b |
Means carrying different superscripts within the same column are significantly different at (P<0.05) based on (least significant difference) LSD.
Effect on Serum Levels of Testosterone and Luteinizing Hormone (LH)
Our data displayed a significant reduction in serum level of testosterone hormone in group treated with crude extract of Euphorbia peplus compared with control one. Table 3 showed a significant decrease in serum level of LH in the treated group compared to control one.
Table 3: Effect of extract of Euphorbia peplus at dose level of 500mg kg. b.wt. for 65 days in mature male albino rats on the serum levels of testosterone and luteinizing hormone (LH), (Means + SE)
|
Parameters |
||
|
Groups |
Testosterone Hormone (mg/ml) |
Luteinizing Hormone (Iu/ml) |
|
Treated |
0.79±0.04b |
1.71±0.42b |
|
DMSO |
0.95±0.30a |
5.23±0.17a |
|
Control |
0.98±0.05a |
6.41±1.53a |
Means carrying different superscripts within the same column are significantly different at (P<0.05) based on (least significant difference) LSD.
Table 4: Effect of extract of Euphorbia peplus at dose level of 500mg/kg b.wt. for 65 days in serum mature male albino on GGT, ACP, total protein, (Means + SE)
|
Parameters |
|||
|
Groups |
Gamma glutamyl transferase (IU/L) |
Acid phosphatase (IU/L) |
Total protein (gm/dL) |
|
Treated |
53.62+5.03b |
8.61+0.25b |
6.10+0.12b |
|
DMSO |
28.66+1.39a |
11.79+0.40a |
6.88+0.12a |
|
Control |
34.90+2.73a |
12.72+0.40a |
6.88+0.12a |
Means carrying different superscripts within the same column are significantly different at (P<0.05) based on (least significant difference) LSD.
Effect on Liver Enzymes Gamma Glutamyl Transferase, Acid Phosphatase and Total Protein
The obtained results revealed that treated group with crude extract of Euphorbia peplus at dose level 500mg/kg b.wt showed a significant increase in liver enzymes including GGT compared to control one but there is a significant decrease in both ACP and total protein in the treated group compared to control one (Table 4).
Effect on Serum Levels of Superoxide Dismutase (SOD), Glutathione Reductase (GR) and Catalase
The obtained data revealed that treated group with Euphorbia peplus extract showed a significant decrease in superoixide dismutase, glutathione reductase, and Catalase enzyme as compared to control one (Table 5).
Table 5: Effect of crude extract of Euphorbia peplus at dose level of 500mg/kg b.wt. for 65 days in mature male albino rats on the superoxide dismutase (SOD), glutathione reductase (GRD) and Catalase levels, (Means + SE)
|
Parameters |
|||
|
Groups |
Superoxide dismutase (SOD) (u/mL) |
Glutathione reductase (GRD) (u/L) |
Catalase (u/mL) |
|
Treated |
42.99+0.90b |
5.74+0.27b |
43.62+2.27b |
|
DMSO |
86.97+4.27a |
10.41+0.67a |
62.16+6.07a |
|
Control |
80.97+0.91a |
9.74+0.34a |
70.39+3.01a |
Means carrying different superscripts within the same column are significantly different at (P<0.05) based on (least significant difference) LSD.
Effect on Testes and Liver DNA Fragmentation
The present study provoked an obvious DNA fragmentation in both testicular and hepatic tissue after treatment with extract of Euphorbia peplus twice weekly for 65 days compared to control group (Table 6).
Table 6: Effect of Euphorbia peplus extract on DNA fragmentation at dose level 500mg/kg b.wt. for 65 days in mature male albino rats, (Means ± SE).
|
Parameters |
||||
|
Groups |
Liver Intact DNA (%) |
Liver Fragmented DNA (%) |
Testis Intact DNA (%) |
Testis Fragmented DNA (%) |
|
Treated |
54.1±1.27b |
45.5±1.27b |
62.0±2.05a |
35.0±0.60a |
|
DMSO |
73.8±1.08a |
26.17±0.58a |
83.0±1.15a |
15.7±1.67c |
|
Control |
76.2±1.71a |
22.93±1.04a |
78.0±19.1a |
21.9±1.10b |
Means carrying different superscripts within the same column are significantly different at (P<0.05) based on (least significant difference) LSD.
Histopathological Findings
After 65 days of treatment with crude extract of Euphorbia peplus in mature male albino rats our study revealed that:
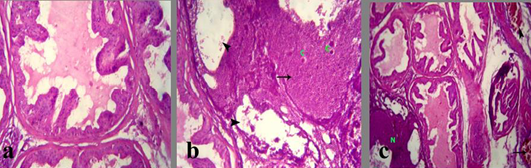
The testis in group treated with crude extract of Euphorbia peplus at dose level 500mg/kg B.wt revealed testicular degeneration which represented by atrophied irregular seminiferous tubules, vaculation of its lining epithelia in complete spermatogenesis. The latter was represented by empty lumina of such tabules from spermatozoa (Figure 2b and c). Sometimes, sloughing of the germ epithelium was detected in the lumina of the seminiferous tubules with formation of few spermatocyte and spermatid giant cells. Interstitial edema, vaculation of Leyding cells and congested blood vessels were also noticed (Figure 2d).
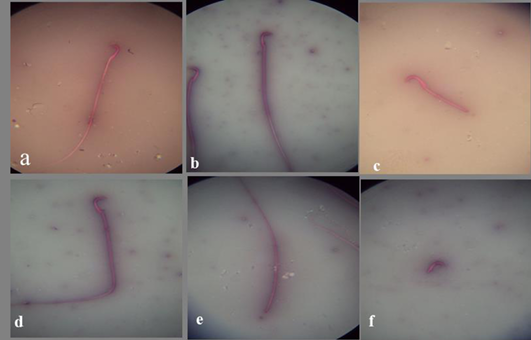
a) Spermatozoon of the control mature male albino rat showing normal sperm; b) Spermatozoon of the DMSO mature male albino rat showing normal sperm; c) Spermatozoon of treated mature male albino rat orally administrated crude extract of Euphorbia peplus at dose level of 500mg/kg b.wt. twice weekly for 65 days showing detached tail; d) bent tail; e) detached head; f) detached tail
a) Section in Testis of Control mature albino rat showing normal germ cells of seminiferous tubules and Leydig cells; b, c) Section in testis of treated mature male albino rat orally administrated crude extract of Euphorbia peplus at dose level of 500mg/kg b.wt twice weekly for 65 days showing testicular degeneration which represented by atrophied irregular seminiferous tubules and vacuolation of its lining epithelia (arrows) besides incomplete spermatogenesis (*) and interstitial edema (arrowhead); d) interstitial edema (arrow), vacuolation of leydig cells and congested blood vessels; H&E (Bar=100µm).
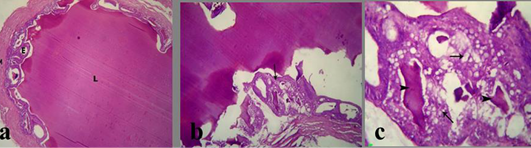
The prostate glands in group treated with crude extract of Euphorbia peplus revealed an area of necrosis, corpora amylacea and cystic dilation of acini without secretory fluid (Figure 3b). Congested blood vessels with or without hemorrhage were seen beside the necrotic area (Figure 3c). Also, the seminal vesicle in treated group showed severe vaculation of the lining epithelium and scanty insipisated secretion (Figure 4b and c). Adenomatous hyperplasia with focal nuclear stratification and apoptosis was also noticed.
a) Section in prostate of Section in Prostate of the control mature male albino rat showing normal lobulation, lining epithelium and fluid in the lumen and acinar spaces; b) treated mature male albino rat orally administrated crude extract of Euphorbia peplus at dose level of 500mg/kg b.wt twice weekly for 65 days showing area of necrosis (arrows), corpora amylacea (C) and cystic dilation of acini without secretory fluid (arrowheads); c) congested blood vessels (arrow) and hemorrhage (arrowhead) besides the necrotic area (N)
a) Section in Seminal vesicle of the control male albino rat showing normal lumen filled with eosinophilic fluid (L), secretory epithelium (E) and muscular coat (M); b, c) treated mature male albino rat orally administrated crude extract of Euphorbia peplus at dose level of 500mg/kg b.wt twice weekly for 65 days showing severe vacuolation of lining epithelium (arrows) and scanty insipisated secretion (arrowheads)
Discussion
Euphorbia peplus is primarily local to Europe and North Africa (Zhi – Qin et al., 2010). Its milky sap has long been used as a remedy for treatment of skin cancer especially non-melanoma skin cancer and the active compounds have been determined to be diterpene esters Ramsay et al. (2011).
In this study we focused on determination the toxic effects of the crude extract of Euphorbia peplus at dose level of 500mg/kg b.wt twice weekly for 65 days on mature male albino rats; including its effect on male fertility, biochemical parameters, and DNA fragmentation and histopathological changes.
The present study revealed that there was a significant reduction in the weights of testis and seminal vesicles in that group treated with Euphrorbia peplus at previously mentioned dose. This result were in agreement with those mentioned by Bataineh et al. (2010) who found that methanolic extract of Euphorbia prostrate while administrated orally for 60 days to male rats did not give rise to any significant losses in their body weight but the weights of their reproductive organs (testis, epididymis, seminal vesicle) declined in a significant manner when compared with the controls. Contrary to our results the effect of methanolic extract of Euphorbia hirta leaf was studied on mice at dose level of 5000 mg/kg which showed no significant statistical difference between body weight, relative (%) absolute (g) reproductive organ weights from treated and untreated groups (p>0.05) also, gross and microscopic examination of the sexual organs, tissues revealed no difference between control and treated mice (Wan et al., 2013). Semen picture analysis in the current work implied a significant decrease in the sperm cells concentration and percentage of sperm motility. Also there is an increase in the percentage of sperm abnormalities in the group treated with crude extract of Euphorbia peplus compared to control one.
Our result about semen picture were in harmony with those mentioned by Oyeyemi et al. (2009) who found that the effect of Euphorbia hirta at a dosage of 400 mg/kg body weight for 14 days illustrated significant differences in the semen characters. On this respect Hameed et al. (2010) reported that Euphorbia prostrata minimized the fertility of male rats by 100% in addition the protein, glycogen and cholesterol contents of the testis, fructose in the seminal vesicle and protein in the epididymes significantly reduced. Histopathological findings of the testes displayed vaculation at the primary spermatocyte stage. The extremely reduced seminiferous tubular diameter picture as reflected in a reduction of sperm motility compared to control group, on alike manner our histopathological findings in the present experiment revealed testicular degeneration which represented by atrophied irregular seminiferous tubules, vaculation of its lining epithelia in complete spermatogenesis which represented by empty lumina of such tubules from spermatozoa, sometimes, sloughing of the germ epithelium was detected in the lumina of the seminiferous tubules with formation of few spermatocyte and spermatid giant cells. Interstitial edema, vaculation of Leydig cells and congested blood vessels were also noticed Figure 2d, also, the seminal vesicle in treated group showed sever vaculation of the lining epithelium and scanty secretion.
In harmony with Mathur et al. (1995) mentioned that Euphorbia hirta at those level of 50mg/kg b.wt. reduced the sperm motility from 80% to 47.5% and intensity of Cauda epididymal and testis & sperm suspension significantly produce ultimately 100% infertility. The previous result may be confirmed by the disturbance in the hormonal levels of testosterone and LH in this study.
Also, the before mentioned results may be confirmed by our histopathological findings which showed testicular degeneration represented by atrophied irregular seminiferous tubules, vaculation of its lining epithelia within complete spermatogenesis.
The present study demonstrated a significant alteration in the serum levels of both testosterone and luteinizing hormone in group treated with crude extract of Euphorbia peplus. The reduction in testosterone level in the current study may be confirmed by our histopathological findings which revealed focal testicular degeneration in some seminiferous tubules with reduction in the spermatogenesis.
The present study also revealed a significant disturbance in liver enzymes (GGT, ACP and total protein) in group treated with crude extract of Euphorbia peplus.
These consequences are in partial conformity with those findings of Adedapo et al. (2004) who investigated that the toxic influences of suspected poisonous plants of the genus Euphorbia (Euphorbia balsamifera, E. heterophyllal, E. hirta., E. hyssopifolial and E. Lateriflora) which were evaluated in albino rats using aqueous extracts for 14 days revealed changes in hematological as well as biochemical parameters which indicate toxicosis as E. Lateriflora, E. hyssopifolial caused a significant elevation (P<0.05) in the level of total protein. The remaining Euphorbia balsamifera, E. heterophyllal caused an insignificant increase, while all the plants caused a significant elevation in the level of albumin. The contrary was the case for globulin. All the plant also brought about a significant elevation in the levels of plants (ALT and AST). Similarly to Atlam (2000) who stated that B. Alexandrina snails after acute exposure of E. peplus should lower activity of both acid & alkaline phosphatase. These results are in harmony with those mentioned by Suganya et al. (2010) who stated that oral administration of ethanolic extract of Euphoriba hirta at dose level 400mg/kg b.wt. showed decrease in level enzyme (ACP, ALP & total protein) in treated rat than in control group.
Regarding the effect of crude extract of Euphorbia peplus on antioxidative enzymes, our study revealed that there was a significant decrease in the superoxide dismutase enzyme, catalase and glutathione reductase enzymes in the treated group compared with the control one. These result are in harmony with those finding of Ashish et al. (2010) who found that ethyl extract of E. hirta at dose level 200mg/kg b.wt. revealed a significant decrease in Glutathione, Superoxide-dismutase, catalase and lipid peroxidation respectively. The previous results were consistent with those recorded by Farong et al. (2006) who found that the extract from Euphorbia Kansui reduces the antioxidant activities of superoxide dismutase. Also our data are in correspondence with the findings of Hsieh et al. (2011) which revealed that Euphorbia antiquorum (EA) at dose levels (2 µg/ml) for 4 hrs. induced, decrease in superoxide dismutase (SOD) and protein levels of Hela cells. Contrary to our results Bigonia and Rana (2009) reported that saponin separated from E. neriifolia leaf possess good hemolytic and in-vitro antioxidant activity. In their study assumed to investigate the effect of sub-acute administration of E. neriifolia leaf extract (400 mg/kg) on some haematological, biochemical, histological and antioxidant enzymes condition of rat liver and kidney following 21 and 45 days treatment with E. neriifolia, free radical scavenging activity and histopathology was done on liver, and kidney samples which depicted an extremely significant (P<0.001) increase in liver and kidney SOD long with liver catalase.
Concerning the effect of crude extract of Euphorbia peplus on DNA, the present work revealed DNA damage and fragmentation in both testicular and hepatic tissue in the treated group. Similarly Hamsa et al. (2013) reported that the latex milk of Euphorbia antiquorum induced a maximal DNA damage in spleen cells. The extent of DNA damage by fragmentation was analyzed in the spleen cells exposed to latex milk of Eurphorbia antiquorum. On this respect Ozlem and Tulay (2012) reported that Euphorbia platyphyllos extracts at dose level (200 g) induced significant DNA damage moreover E. platyphyllos possess antioxidant properties and provoke DNA fragmentation. Meanwhile Wan et al. (2011) reported that the latex of Eurphorbia antiquorum (2 µg/ml) induced apoptosis which was characterized by morphological change and DNA fragmentation in hela cells.
Conflict of Interest
The authors declare that they have no competing interests.
Authors contribution
GGM, ATM and AHA participated in designing and coordination of the manuscript, carried out the experiment and drafted the manuscript . FHK and EME carried out the statistics and tabulated the data. All authors approved the final manuscript.
Acknowledgement
All the authors of the manuscript thank and acknowledge their respective Universities and Institutes.
References