Advances in Animal and Veterinary Sciences
Research Article
The Impact of Dog Feeding on their Aggressiveness
Kocis Timea Andrea1*, Groszler Astrid-Simone2, Zarcula Simona1, Petruse Cristina1, Brudiu Ileana1, Cărpinișan Liliana1, Ţibru Ioan1
1Faculty of Veterinary Medicine; 2Faculty of Agriculture, Banat’s University of Agriculture and Veterinary Medicine “King Michael I of Romania” from Timișoara (BUASVMT), 119 Calea Aradului, Timișoara 300645, Romania.
Abstract | The current study aimed to determine the serum serotonin in 10 dogs, separated in two different emotional states (calm, aggressive). According to the specialty literature, at the aggressiveness moment, the serotonin value drops. The aggressiveness manifestation in dogs is induced by several factors, among which feed occupies a special role, hypo-hyper-protean ratios influencing the behaviour. The results indicate that individuals fed with hyper-protean ratios presented lower serotonin values as compared to dogs receiving hypo-protean ratios.
Keywords | Aggressiveness, Dog, Feed, Serotonin, Tryptophan
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 03, 2015; Revised | July 23, 2015; Accepted | July 25, 2015; Published | July 31, 2015
*Correspondence | Kocis Timea Andrea, Banat’s University of Agriculture and Veterinary Medicine “King Michael I of Romania” from Timișoara, Calea Aradului, Timișoara, Romania; Email: dea16_tim@yahoo.com
Citation | Andrea KT, Astrid-Simone G, Simona Z, Cristina P, Ileana B, Liliana C, Ioan Ţ (2015). The impact of dog feeding on their aggressiveness. Adv. Anim. Vet. Sci. 3(9): 503-506.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.9.503.506
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Andrea et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The effects of feed composition on mammal behaviour represent a subject discussed for many years in the scientific world. With dogs, there are attempts to connect the feed protein content, the tryptophan metabolism with aggressive behaviour.
The behaviour score of dominant aggressiveness was higher in dogs receiving hyper-protean ratios not supplemented by tryptophan. Hypo-protean ratios supplemented by tryptophan were associated with a lower aggressiveness score as compared to individuals ingesting feed without a tryptophan supplement.
Supplementing hypo- as well as hyper-protean dog feed with tryptophan with dogs displaying dominant and territorial aggressiveness leads to an intensity reduction in manifesting this behaviour.
Hypo-protean diets including high carbohydrate content change the tryptophan plasma concentration, producing its increase, as opposed to the level of other large neutral amino-acids, with which it competes at the hematoencephalic barrier. Protean rich diets have low tryptophan content, being rich in large neutral amino-acids, so that the tryptophan plasma concentration is not enough to sensitize the carrier and to cross the hematoencephalic barrier (De Napoli and Nicholas, 2000).
L-Tryptophan (tryptophan, Figure 1), an indole propionic acid, is one of 8 essential amino-acids and since its content in the organism is the lowest, as compared to the other amino-acids, it may play a percentage limitation role regarding protein synthesis (Sainio and Pulkki, 1996).

Figure 1: Tryptophan structural shape
In the mammal brain, tryptophan is the precursor of serotonin (5-hidroxitriptamine), influencing a series of behaviours: aggressiveness, impulsivity, feed selection, sexual behaviour and reaction to pain, serotonin being also involved in disposition control.
Inside the organism, serotonin is obtained through tryptophan hydroxylation and decarboxylation, and direct tryptophan decarboxylation leads to tryptamine (Figure 2) (De Napoli and Nicholas, 2000).
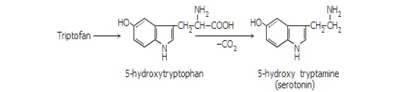
Figure 2: Tryptophan hydroxylation
Quantitatively, the most important way to metabolise tryptophan, after protein synthesis, is the kynurenic way, which is over 90% responsible for tryptophan metabolization. Two enzymes initiate this way, tryptophan-2, 3-dioxygenase present in the liver and indolamine-2, 3-dioxygenase, which can be found in a variety of tissues (Bertrand, 2010).
Tryptophan is the only amino-acid bound by plasma albumin, and only a part of the tryptophan being available for brain absorption (Sainio and Pulkki, 1996).
Under certain circumstances, as a result of free plasma tryptophan level increase, these factors (mentioned above: tryptophan presence in feed, its availability, the presence of other amino-acids) lead to a serotonin increase in the brain. Another factor with a greater effect regarding tryptophan brain absorption is the plasma level of other large neutral amino acids (LNAA) (phenylalanine, tyrosine, leucine, isoleucine, valine, histidine, methionine and threonine) (De Napoli and Nicholas, 2000).
All these large neutral amino acids are competing for access to the carrier in order to reach the brain. Each amino acid level in the brain will depend not only on its plasma level, but also on the plasma level of other amino-acids it competes with at the hematoencephalic barrier (Walz et al., 2013).
In the pineal organ, tryptophan is transformed in melatonin (hormone) through a process triggered by the same two reactions one can find in serotonin production within terminal nerves (5-hydroxylation, followed by decarboxylation) (Bertrand, 2010). During the day, a large part of this serotonin is deposited in a form inaccessible either for melatonin producing enzymes, hydroxyindole-o-metyltransferase or for mono-amine oxidase. Once evening falls, within sympathetic nerves, the heightened norepinephrine secretion of the pineal gland makes it possible for this type of serotonin to become accessible to metabolism and a similar major N-acetyltransferase activation, determines a great part of the serotonin to be metabolized through melatonin conversion. There is little information referring to possible effects of pineal gland tryptophan level changes on the melatonin synthesis (Walz et al., 2013).
MATERIALS AND METHODS
This study included 10 dogs, 7 crossbreeds and 3 pure breeds, from which blood samples were taken at two different moments. After setting up a branula, blood samples were taken from the cephalic vein, in 6 ml vacutainers, without anticoagulant. The first moment of blood sampling was considered moment 0 (M0), at a 5 minute interval after setting up the branula, and moment 1 (M1), after the dog was provoked to become aggressive and blood samples were taken.
The dogs involved in the study were grouped in two lots, all of them receiving dry feed from the same supplier for 6 weeks. 5 individuals received a hyper-protean ratio containing 28% protein, while the other 5 dogs were given feed containing 25% protein and a tryptophan supplement.
The harvested blood samples were kept at room temperature for 2 h, and afterwards were centrifuged at 3500 rotation/min for 5 min. The expressed serum was collected with Pasteur pipettes and was stored in Eppendorf pipettes in the freezer at -40°C until the serotonin determination. The serum serotonin determination was carried out with a MikroWin 2010, Version 5 device, using the Serotonin ELISA Enzyme Immunoassay for the Quantitative Determination of Serotonin in Serum, Plasma and Urine kit, from the DLD Diagnostika firm in Hamburg, Germany.
RESULTS
The average value of the first moment is of 265.74 ng/ml, which is higher than at the aggressiveness moment, when it only reached 146.14 ng/ml. The obtained results are presented in Figure 3 and in Table 1.
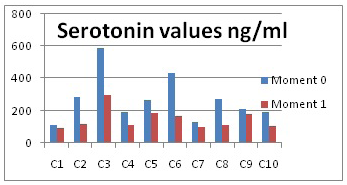
Figure 3: Serotonin values
Table 1: Serotonin values at M0 and M1
|
Dogs |
Moment 0 |
Moment 1 |
||
|
Serotonin value ng/ml |
Animal state |
Serotonin value ng/ml |
Animal state |
|
|
Dog 1 - Romanian mioritic shepherd crossbreed, male, 4 years old, used as a watchdog at a cow farm. |
113.55 |
calm at the moment of branula set-up, showing no sign of discomfort |
95.1 |
became aggressive, causing the serotonin value to drop |
|
Dog 2 - German shepherd crossbreed, male, 1 year old |
281.93 |
calm at the moment of the branula set-up, showing no sign of discomfort |
115.52 |
became aggressive, causing the serotonin value to drop |
|
Dog 3 - Carpathian shepherd crossbreed, male, 3 years old, aggressive, defending its territory. |
583.02 |
opposed the branula set-up operation, making a strong contention by the owner necessary |
298.17 |
aggressiveness moment brought about by a person it dislikes, causing the serotonin value to drop |
|
Dog 4 - mioritic shepherd crossbreed, female, 4 years old, watchdog. |
188.74 |
difficult branula set-up moment, showing signs of anxiety and discomfort |
111.73 |
barking alongside the fence when someone was passing by in the street |
|
Dog 5 - mioritic shepherd crossbreed, female, 5 years old, presenting territorial aggressiveness. |
261.93 |
calm at the moment of branula set-up, showing no sign of discomfort |
181.64 |
became aggressive, causing the serotonin value to drop |
|
Dog 6 - German shepherd, female, 3 years old |
42.3 |
calm at the moment of branula set-up, showing no sign of discomfort |
167.5 |
became aggressive, causing the serotonin value to drop |
|
Dog 7 - German shepherd, female 6 years old |
128.35 |
calm at the moment of branula set-up, showing no sign of discomfort |
98.3 |
became aggressive, causing the serotonin value to drop |
|
Dog 8 - crossbreed, male, 4 years old |
272.13 |
calm at the moment of branula set-up, showing no sign of discomfort |
110.13 |
became aggressive, causing the serotonin value to drop |
|
Dog 9 - crossbreed, male, 4 years old. |
210.05 |
calm at the moment of branula set-up, showing no sign of discomfort |
180.20 |
became aggressive, causing the serotonin value to drop |
|
Dog 10 - Boxer, male, 2 years old |
188.42 |
calm at the moment of branula set-up, showing no sign of discomfort |
103.16 |
became aggressive, causing the serotonin value to drop |
DISCUSSIONS
The results obtained in this study are in accordance with the results obtained by other researchers, the serotonin value at the aggressiveness moment being lower than that reached at the reference moment (Rosado and Leon, 2010).
The results obtained were interpreted with the help of the Student Test, undertaking a comparison between the averages of the two moments. It was established that there is a significant difference between moment 0 and moment 1, p<0.05 (p=0.02).
The lowest value was determined with dog 7, 98.3 ng/ml and the highest value at the aggressiveness moment was 298.17 ng/ml. This difference between the highest and lowest values is accounted for by individual variability, that is, each dog will respond differently to environment stimuli.
Dogs 1, 7 and 10 showed the lowest plasma levels, manifesting dominance aggressiveness, a fact which is in accordance with the received ratio (hyper-protean), as compared to dogs 3, 5 and 9, which presented the highest serotonin values and manifested territorial aggressiveness. This fact is also in accordance with the ratio they received (hypo-protean).
Similar results were obtained by Rosado and Leon (2010), in a studio. Based on these results, he classified dogs in four categories, depending on the manifested aggressiveness and the serum serotonin value reached:
The serum serotonin concentration in the control group of 387.4 ng/ml, is a result similar to the data published by Chen et al., (1993), measuring lower serotonin values in aggressive dogs, 278.5 ng/ml, which indicates a reversed correlation between the serotonin concentration and aggressiveness in several animal species, including dogs.
In yet another research carried out by Cakiroglu et al., (2007), dogs manifesting aggressiveness towards people / other dogs showed a 12ng/ml serotonin value, as compared to the control group which showed a 32.5 ng/ml serotonin value. These large differences between the two study groups can be explained by the different work methodology (Cakiroglu et al., 2007).
Given the fact that the lowest serotonin values were obtained in dogs manifesting defensive aggressiveness, it is presumed that fear and anxiety is the basis of these behaviours. De Napoli and Nicholas (2000), showed that supplementing feed with tryptophan (a serotonin precursor), decreases the frequency of dominant and territorial aggressive behaviour.
Of the studied dogs, the ones with the lowest serotonin values (1, 7 and 10) manifested dominant aggressiveness, a fact corresponding with their hyper-protean ratio.
The highest serotonin values correspond to dogs fed with hypo-protean ratios, which manifested territorial aggressiveness.
The aggressive behaviour is correlated with the hyper-protean alimentation due to the fact that this type of ratio insures a higher large neutral amino-acid percentage (phenylalanine, tyrosine, leucine, isoleucine, valine, histidine, methionine and threonine), but a too low tryptophan quantity, which leads to a reduced serotonin production.
ACKNOWLEDGEMENTS
This paper was published under the frame of European Social Fund, Human Resources Development Operational Programme 2007-2013, project no. POSDRU/159/1.5/S/132765.
Authors’ contributions
This article is part of a broader study, the PhD thesis, entitled “Social Dog Behaviour: Aggressiveness”, written by the undersigned, PhD student Kocis Timea Andrea. Rest of the authors assisted equally in completion of the study.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES





