Advances in Animal and Veterinary Sciences
Research Article
Effect of Sarcocystosis Infection on Cytogenetic Peripheral Blood Lymphocytes in Ewes
Amer Murhum Abd AL-Amery
Department of Parasitology, Faculty of Veterinary Medicine, Baghdad University, Baghdad, Iraq.
Abstract | Aim of this study was to estimate the potential genotoxoicity of sarcocystiosis on cytogenetic of infected ewes lymphocystes by using different cytogenetic test. Forty ewes infected with sarcocystiosis and 20 healthy were collected as an uninfected group. Lymphocytes from peripheral blood of infected and uninfected ewes were used for culture and subsequent cytogenetic studies. Cytogenetic analysis was used as a marker to reflect the genotoxic effect of sarcocystosis parasite on the lymphocytes. The mean Mitotic Index (MI) of infected ewes was significantly lower (P≤ 0.05) than of control group, while mean replicative index (RI) of infected ewes was non-significant as compared with uninfected group. Sister Chromatid Exchange (SCEs) and Chromosomal Aberrations (CAs) showed significant (P≤ 0.05) increase in infected ewes as compared with control group.
Keywords | Sarcocystosis, Cytogenetic analysis, Mitotic index, Replicative index, Chromosome aberration
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 19, 2015; Revised | July 04, 2015; Accepted | July 05, 2015; Published | July 14, 2015
*Correspondence | Amer Murhum Abd AL-Amery, Baghdad University, Baghdad, Iraq; Email: m.murhum@yahoo.com
Citation | AL-Amery AMA (2015). Effect of sarcocystosis infection on cytogenetic peripheral blood lymphocytes in ewes. Adv. Anim. Vet. Sci. 3(9): 473-477.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.9.473.477
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 AL-Amery. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sarcocystis species cause Sarcocystosis which is an intracellular protozoan parasite in the phylum Apicomplexa (Levine, 1986). There are two type of the host in the life cycle of this parasites definitive and intermediate host (Fayer and Leek, 1979). There are two phase in the infection with sarcocystosis intestinal phase occurs in the definitive host and tissue phase which seen in the intermediate host. More than hundred species of Sarcocystis are parasites of domestic and wild animals, many of these infections are asymptomatic, particulary in the definitive host (Beaver et al., 1979). Several species of Sarcocystis parasites infected Sheep like S. tenella (S. ovicanis), S. arieticanis, S. gigantea (S. ovifelis) and S. medusiformis where dogs (for first two species) and cats (for last two species) are the final hosts (Aiella et al., 1998). In general, the Sarcocystis spp., by canidae or primates is more pathogenic than those transmitted by felids. Intermediate hosts show symptoms of fever, anemia, weight loss, reduction of wool quality and milk yield, abortion and CNS. Signs are mainly death in many cases. In the final hosts diagnosis of the Sarcocystosis depened on detection the sporocyst by feacal analysis , and in the intermediate hosts the diagnosis done by investigated the asexual stages as cyst or their contains (cystizoites) in the muscles by several trichinoscopy (Scott, 1930; Daoud, 1976), Peptic digestion technique for the muscles (Jacobs et al., 1960). Cytogenetic analysis is a widely employed indicator system for induced mutations. It allows objective evolution of insults to the genetic martial and is a method that permits visual analysis of chromosome damage (Sinues et al., 1990; Tucker and Preston, 1996) +lymphocyte of affected ewes by using blood samples.
Materials and methods
Sixty samples (weight range between 15-20 gm) of ewes muscles were collected from Shulla abattoir in Baghdad province, of samples, and examined for the presence of bradyzoites Sarcocystis species by Peptic digestion by using digestion solution (pepsin 1.3 gm, 2.5 gm Nacl and3.5 ml HCL which dissolve in 500 ml distilled water) these samples were incubated for 30 min. at 40°C, sediments were smeared on slides and staining by Giemsa stain, followed by microscopic examination (10x and 20x), to diagnosis of Sarcocystosis (Jacobs et al., 1960). Pepsin digestion test isolated positive samples from negative samples which considered as control group.
Mitotic Index Assay (MI) and Chromosome Aberration (CA)
Five millilitres of blood samples in heparinized tube were collected from jugular vein of 60 ewe before slaughter. 0.4 ml of the heparinized blood samples, obtained from the subjects, were placed in sterile culture tubes containing 5 ml of Qunitum medium. Gently shaking the contents of each culture tube and then incubated in a slanted position at 37°C for 72 h. After 70 h of incubation, 0.1 ml of colcemid solution (10 μg/ ml) was added to each culture tube and mixed by shaking gently. After replace of 72 h of incubation, the tubes were centrifuged at 2000 rpm for 4 min. and the supernatant was discarded. The pellet was resuspended using 10 ml of hypotonic solution (0.075M KCl) and the tubes were incubated at 37°C for a further 4 min. After the tubes were centrifuged at 2000 rpm for 4 min., the supernatant discarded, and the pellet was resuspended using 10 ml of fresh fixative solution (methanol and acetic acid, 3:1). The tubes were centrifuged at 2000 rpm for 4 min. and the supernatant was discarded. This procedure was repeated 3 times. The pellet was resuspended and 0.5-1 ml of fresh, cold fixative solution was added to the tubes. Then 3 or 4 drops of the cells suspension were dropped on to a cold wet glass slide. Dried slides were stained with 5% Giemsa stain. Mitotic index (MI) was determined as a percent of the Mitotic cells interphase nuclei in 1000 cells. Replicative index (RI) was determined by counting the number of cells at the first, second and the third in (100) cells at metaphase (Rooney and Czepulkowski, 1986).
Sister Chromatid Exchange (SCE) Assay
To determine the frequency of Sister Chromatid Exchange (SCE) in the lymphocytes, 1–2.5 ml sample of peripheral blood from each individual was added to 5 ml of chromosome medium supplemented with 5 μg/ml of phytohaemagglutinin. BrdU (5–bromo–2–deoxyuridine,) was then added to each culture (10-4 M final concentration) and the cultures were incubated in complete darkness for 72 h at 37°C. Colchicine (final concentration 0.5 μg/ml) was added to each culture during the last 2 h of the incubation to block the cells in the metaphase stage of mitosis. Upon completion of the incubation, each cells culture was subjected to hypotonic treatment by adding 8 ml of 0.075 M KCl and was maintained for 30 min at 37°C. Three repetitive fixations of each cells suspension were then performed with cold methanol/acetic acid (3:1, v/v). An aliquot of each cell suspension was then dropped onto a cold slide. The slides were dried at room temperature, and then kept in the dark for 3 days after which they were stained using acridine orange method. The number of SCEs was counted in at least 50 metaphases in each sample from each individual. The number of SCEs per metaphase was scored for each individual (Rooney and Czepulkowski, 1986).
Slides Stain
Slides were stain with 0.1 mg/mL acridine orange in dH2O for 5 min. at room temperature, then washed extensively for 2 min. under running dH2O tap water, slides incubated for 1 min in Sorenson Buffer, pH 6.8 (0.1M Na2HPO4, 0.1M NaH2PO4) and then mount in Sorenson Buffer, pH 6.8. Slide view immediately under FITC filter and count reciprocal exchange events (Rooney and Czepulkowski, 1986).
Statistical Analysis
All data obtained were analysed statistically. Means differences were estimated with T-test using SPSS at level (P> 0.05) (Snedecor and Cochran, 1989).
Results and Discussion
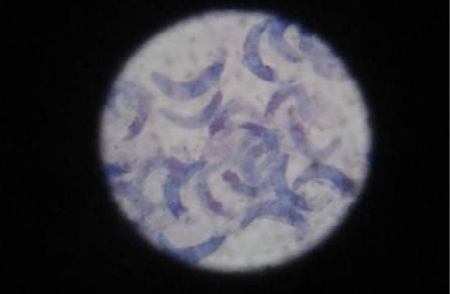
Cystizoit of ovine sarcocystosis in 40 out of 60 (66.6%) were found during carcass inspection. Banan shaped bradyzoites in the digested muscle tissue are shown in (Figure 1). The present study raveled the high rate with ovine sarcocystosis. This finding agreed with previous studies in Iraq (AL-Delemi, 1992; AL-Bayati, 2008). The study was indicated high contacted among intermediate and final hosts. This observation was confirmed by other researches (Rommel, 1985).
Table 1: The Means and SE of Mitotic Index, Replecative Index and Sister Chromatid Exchanges in ewes
|
Ewe |
Mitotic Index MI% |
Cell Cycle Progression C.C.P |
Replicative Index R.I% |
Mean S.C.E/Cell |
||
|
M1% |
M2% |
M3% |
||||
|
Sarcocystosis infected ewes (40) |
1.95 ± 0.096B |
79.28 ± 1.1 A |
15.70 ± 0.78 B |
5.1 ± 0.45 B |
108.41 ± 2.53 |
8.63 ± 0.16 A |
|
Control (20) |
5.95 ± 0.25 A |
53.9 ± 2.46 B |
27.75 ± 1.03 A |
18.4 ± 1.7A |
109.95 ± 1.38 |
1.53 ± 0.09 B |
The different letters refer significant between infected and non-infected group at (P<0.05)
Table 2: The Means and SE of Chromosomal Aberrations in ewes
|
Ewes |
Chromosomal Aberration |
Total CA |
Total CA/100 |
|||||
|
G´ |
G˝ |
B´ |
B˝ |
Deletion |
SF |
|||
|
Sarcocystosis in fected ewes (40) |
15.2± 0.41 A |
4.90± 0.42 A |
4.95± 0.37 A |
2.30± 0.19 A |
1.20 ± 0.13 A |
1.06 ± 0.10 A |
29.45 ± 1.26 A |
0.29 ± 0.01 A |
|
Control (20) |
4.20± 0.24 B |
1.20± 0.12 B |
0.70± 0.16 B |
0.45± 0.15 B |
0.15 ± 0.08 B |
0.20 ± 0.09 B |
5.95 ± 1.02 B |
0.70 ± 0.01 B |
The different letters refer significant different between groups at (P<0.05); G´: chromatid gap; G˝: chromosomal gap; B´: chromatid break, B˝: chromosomal break, SF: simple fragment
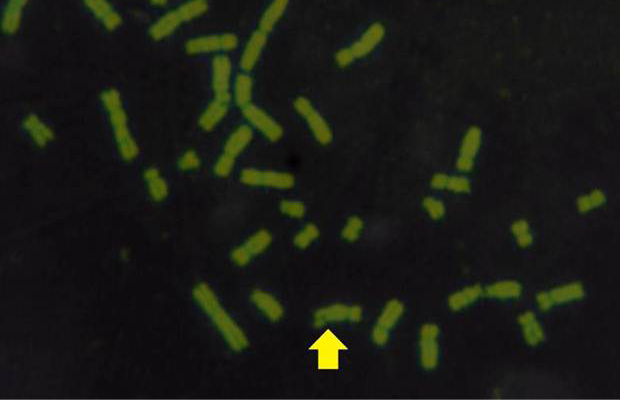
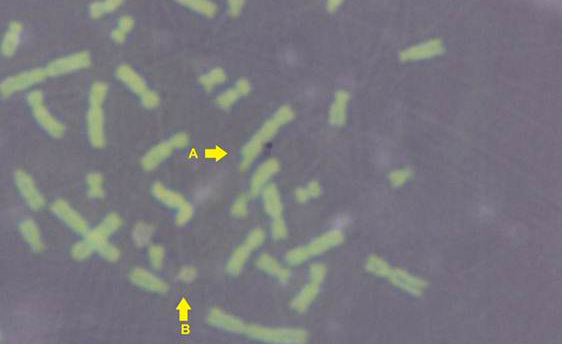
The results obtained from mitotic index, micronuclei assay, sister chromatid exchange and chromosomal aberrations are used to detect DNA damage among ewes infected with sarcocystis spp. The mean Mitotic Index (MI) of infection with sarcocystosis of ewes was significantly (p≤ 0.05) lower than of controls group, and 1.95, 5.95 in infected and control respectively (Table 1), while the mean of SCEs were significantly (p≤ 0.05) higher than that of control group, mean was 8.63, 1.53 infected and control respectively (Table 1 and Figure 2). The Cell Cycle Progression (CCP), mean replicative index (RI) of ewes were no significant (p>0.05) compared with control group (Table 1).
This study demonstrate the mitotic index which a significant (P≥0.05) decreased and increased in the mean frequency of SCEs. These results were in agreement with Hussein (2014) who found that there was significant decreased in MI. and increased SCEs, in both women and ewes infected with T. gondii. On other hands AL-Khafajy (2004) reported that there was significant decreased in MI. in women infected with T. gondii. Researcher such as AL-Ubaidi (2002) found that the mean of MI of patients with hydatid disease in response to the Phytoheamagglutinine PHA was significantly lower than those of healthy controls. This might indicate an immune unresponsiveness state against the parasite Ecchinococcus granulosus.
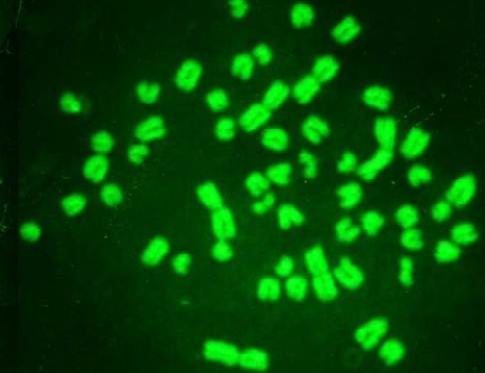
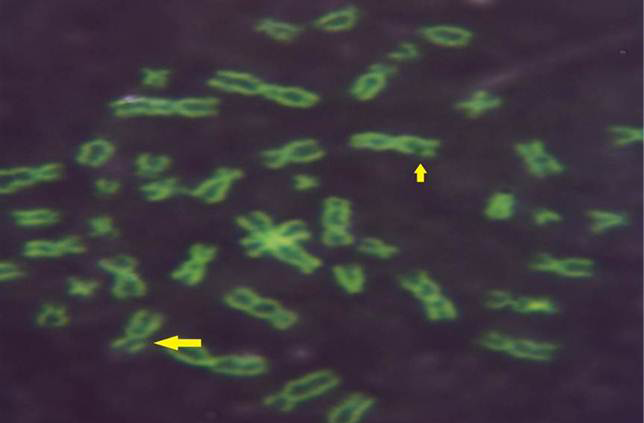
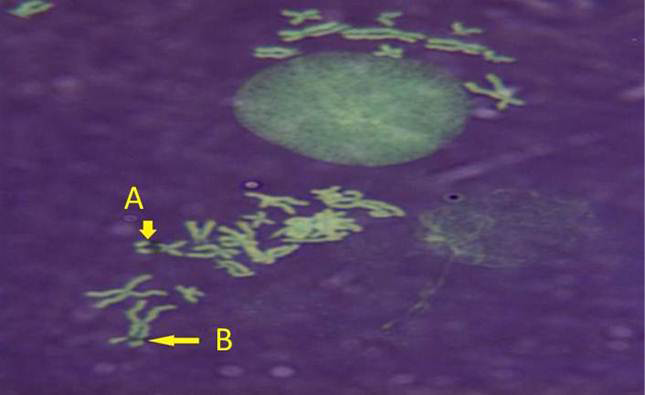
While Baqir et al. (2001) shown lowering MI and RI manifest the decreasing of lymphocytes proliferation. This decreasing extends to non-parasite antigen when lymphocytes responded to PHA. PHA significantly lowers response than in normal healthy control. This may indicate an immune unresponisveness state of immune against the parasite. The genome stability in humans is detected by used SCE test and the main livestock species (Chaganti et al., 1974; Ciotola et al., 2005), to discover DNA damage caused by a variety of natural and artificial chemical compounds (Perucatti et al., 2006). The Chromosomal Abberations (CAs) of ewes were significantly (P≤ 0. 05) higher than that of controls group, it was 0.295, 0.700 in infected and control ewes respectively. The types of CAs which have been observed are chromatid gap, chromosomal gap chromatid break, chromosomal break, simple fragment and deletion (Table 2, Figure 3, 4, 5 and 6).
These results were in agreement with AL-Delemi (2005) who found that there was a significant increase in SCE and CAs in lymphocytes of cows infected with Fasciola gigantica. Also AL-Nsary et al. (2010) suggested that there is significantly increased in SCE and CAs in lymphocytes of human infected with Schistosoma mansoni. CAs are considered damage of chromosomal by change in chromosome structure or chromosome number. Many agents induce visible chromosome damage after the cells undergo around of DNA replication. However, damaged cells may not survive for more than one or two cell cycles after aberration have been induced (Catherine et al., 1998). The frequency of chromosomal aberrations like gaps, breaks, fragments and deletion which were recorded in the present study were both of chromatids and chromosome aberrations. Chromosome abnormalities are considered to be one of the most important cytogenetic parameters for the manifestation of genotoxicity. Brogger et al. (1990) have reported that persons with high frequency of CAs develop cancer twice as often as others. In present study, investigated the sarcocystosis infection showed significantly increased CAS compared with their matched controls.
ACKNOWLEDGEMENTS
My thanks to all staff of Biological Department especially Mr. Muhammad Abdel Wahab in the Ministry of Science and Technology to help me in the performance of cytogenetic analysis and scientific imaging of chromosomes.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
REFERNCES