Advances in Animal and Veterinary Sciences
Research Article
Isolation and Characterization of New Variant Strains of Infectious Bronchitis Virus in Northern Egypt
Sahar Abd El Rahman1*, Markus Hoffmann2, Doerte Lueschow3, Abdel-Fattah Eladl4, Hafez Mohamed Hafez3
1Department of Virology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt; 2Unit of Infection Models, German Primate Center, Gottingen, Germany; 3Institute of Poultry Diseases, Faculty of Veterinary Medicine, Berlin Free University, Berlin, Germany; 4Department of Poultry and Rabbit Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt.
Abstract | Infectious bronchitis virus (IBV) is the causative agent of an acute and highly contagious disease affecting the respiratory, renal and/or reproductive systems of chicken called infectious bronchitis disease. Controlling of this disease needs continuous identification of the circulating strains and genotypes. In the present study, six IBV samples were isolated from different poultry farms located in northern Egypt and propagated in the allantoic cavity of specific pathogen-free embryonated chicken eggs causing curling and dwarfing of embryos at different serial egg passages. The harvested allantoic fluids were titrated by chicken embryo kidney cell cultures selecting suitable viral concentrations for investigating the ciliostasis effect of IBV on tracheal organ cell cultures. In addition, the nucleotide and protein sequences of the IBV spike glycoprotein (S) were used for molecular relationship analysing and construction of phylogenetic trees. All examined viruses induced complete ciliostasis at the fifth day post inoculation. Isolates shared total sequence identities of 99-100% on nucleotide and amino acid levels. The Egyptian strain (Eg/CLEVB-1/IBV/012) showed 99% and the Israeli strain (IS/1494/06 IBV) showed 98% with the collected isolates and recognized as the local strains with the highest total sequence identities and phylogenetic relatedness of all strains available from the Gene Bank. The Italy02 strain of IBV is the nearest relative to the collected isolates with total sequence identities of 79 and 77% at nucleotide and amino acid levels, respectively. The new variant strains currently isolated from the northern of Northern Egypt should be evaluated periodically for developing suitable autogenously based virus vaccines.
Keywords | Infectious Bronchitis Virus, Tracheal Organ Cell Culture, S1 Sequences, Northern Egypt, IBV/Eg/ Mans-1/12
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | April 25, 2015; Revised | May 19, 2015; Accepted | May 19, 2015; Published | May 25, 2015
*Correspondence | Sahar Abd El Rahman, Mansoura University, Mansoura, Egypt; Email: sahar_virol@yahoo.com
Citation | Abd El Rahman S, Hoffmann M, Lueschow D, Eladl A, Hafez HM (2015). Isolation and characterization of new variant strains of infectious bronchitis virus in Northern Egypt. Adv. Anim. Vet. Sci. 3(7): 362-371.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.7.362.371
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Abd El Rahman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
IBV or Avian Coronavirus (AvCoV) belongs to the genus Gammacoronavirus, subfamily Coronavirinae, family Coronaviridae, and order Nidovirales (King et al., 2012). Infectious bronchitis (IB) disease caused by IBV infects mainly the respiratory system and can be extended to other organs as kidney and reproductive system of broiler, layers and breeder chickens of all ages causing major problems of poultry farms worldwide. The IBV genome is a positive sense, single stranded RNA virus of approximately 27 kb in length, encodes four structural proteins: the spike (S), the membrane (M) and the envelope (E) and the nucleocapsid (N) proteins. The (S) glycoprotein is cleaved by proteolysis to S1 and S2 subunits for binding of the virus to the infected cell (Stern and Sefton, 1982). The S1 subunit involves not only in the viral infectivity but also contains a serotype-specific determinant which is located in the hypervariable regions (Cavanagh et al., 1988) and induces neutralizing and haemagglutination-inhibition antibodies (Cavanagh et al., 1988; Koch et al., 1990).
Emerging of new variant serotypes and genotypes of IBV in the field is frequent because of its genomic alterations as mutations and/or recombination of the hyper variable part of the S1 genes which do not cross-protect and therefore hinder complete controlling of the disease by the routinely used vaccination programs (Cavanagh et al., 1992).
The first detection of IBV in Egypt was reported by Ahmed (1954), several IBV strains have been isolated and/or detected from poultry farms in Egypt and identified as Massachusetts, D3128, D274, D08880 and 4/91 (Abdel-Moneim et al., 2006). In addition, novel genotypes called Egypt/Beni-Suef/01, Ck/Eg/Bsu-2/2011, Egypt101-ck and Egypt/F/09 have been recently detected (Abdel-Moneim et al., 2012). The diagnosis and characterization of IBV is based on virus isolation, identification of viral antigens or its biological properties (e.g. hemagglutination activity), and/or by the detection of viral RNA using reverse-transcriptase polymerase chain reaction (RT-PCR), gel electrophoresis and S1 gene sequencing (Cavanagh et al., 1992). The aim of this investigation is to identify and characterize IBV isolates from diseased poultry flocks in Egypt, and compare them to others previously isolated native, neighboring countries and well-known reference strains.
MATERIALS AND METHODS
Samples And Flocks History
Pooled tissue samples (trachea, lung and kidney) were collected from fifteen poultry farms located in two provinces (El-Dakahlyia and Damietta) in Egypt from January to April 2012 (Figure 1). Beside high mortalities (ranging from 40-50%), severe respiratory manifestations like gasping, tracheal rales, sneezing, coughing and oculonasal discharge were observed in chicken. In this study, recent IBV outbreaks were accompanied with other complicating diseases such as chronic complicated respiratory disease (CCRD), Newcastle disease and avian influenza as indicated from signs and lesions. The post mortem examination of freshly dead and sacrificed chickens was carried out. The gross lesions were catarrhal tracheitis, air sacculitis, bronchitis, pneumonia, congested visceral organs, swollen and pale kidneys and ureters distended with urates. Renal lesions and tracheal plugs were recorded in only six flocks as suspected lesions with IBV. The age, vaccination history, clinical signs and post mortem lesions in these poultry farms were reported (Table 1).
Reference Viruses and Antibody
IBV reference strains (Beaudette and H120), provided kindly from colleagues in Veterinary Serum and Vaccine Research Institute, Abbasia, Cairo, were suspended in 0.1 ml sterile phosphate buffer saline (PBS) buffer, stored at -20°C and used as positive controls. Rabbit hyper immune serum prepared against IBV, was prepared according to Abdel-Moneim et al. (2002) and used for titration of IBV viruses by indirect immunofluorescent technique (IFT) for detection of the viral cytopathic effect in chicken embryo kidney primary cell culture (CEK).
Virus Isolation and Titration
The collected organs were pooled and homogenized in sterile saline containing 1,000 IU/mL penicillin and 1.0 mg/ml streptomycin. The homogenates were then centrifuged at 3,000 rpm for 10 min and the supernatants were passaged in embryonated specific pathogen-free (SPF) chicken eggs. Five embryonated SPF chicken eggs of 10 days of age were used for inoculation of each sample via the allantoic cavity. The harvested fluids were inoculated for further two passages (Gelb and Jackwood, 1998).
CEK cells were prepared using 19 days old SPF chicken embryos for virus titration (Villegas and Purchase, 1990). CEK cells were incubated on cover slides of 24 well tissue culture plates and inoculated by 0.1 ml of the harvested allantoic fluids at a multiplicity of infection (MOI) of 0.001. The plates were incubated under slight agitation for an evenly distribution of the inoculum over the cell monolayer at 37°C for one hour. Subsequently, medium containing methylcellulose was added and the cells were re-incubated at 37ºC for 24h. The infected cells were fixed by 3% paraformaldehyde and permialized by 0.2% Triton X-100. Infected cells were incubated with a rabbit hyperimmune serum against IBV for one hour at room temperature followed by incubation with a FITC-conjugated anti-rabbit secondary antibody in dark room for one hour at room temperature. The number of fluorescent foci was quantified by fluorescence microscopy. Viral titers are given as foci forming units per ml (ffu/ml).
Schematic illustration of Egypt and the area where the IBV isolates have been detected (dark gray). The IBV isolates are distributed in El-Dakahlyia and Damietta provinces in the Nile delta area of northern Egypt.
Table 1: The history of the collected samples indicating location, breeds and size of flocks, age, used vaccination and field signs
|
Isolate |
Location |
Breed & Size of flock |
Age |
Vaccination |
Signs & Post mortem lesions |
|
IBV/Eg/Mans-1/12 |
Damietta |
Broiler ( 80000) |
30 days |
Vaccinated (one time, H120 vaccine) |
Respiratory signs of IBV infections and kidney damage with high morbidity and mortality rates accompanied with congested visceral organs |
|
IBV/Eg/Mans-2/12 |
El-Dakahlyia |
Saso (5000) |
45 days |
Vaccinated (one time, H120 vaccine) |
Renal lesions, and chronic complicated respiratory diseases (CCRD) lesions with renal lesions |
|
IBV/Eg/Mans-3/12 |
El-Dakahlyia |
Broiler (40000) |
20 days |
Vaccinated (2 times, H120 vaccine) |
kidney damage with high morbidity and mortality rates, suspected lesions of avian influenza as highly congested wattles and comb and subcutaneous hemorrhage on shanks |
|
IBV/Eg/Mans-4/12 |
Damietta |
Broiler (50000) |
40 days |
Vaccinated (one time, H120 vaccine) |
Severe respiratory manifestations and signs infections |
|
IBV/Eg/Mans-5/12 |
El-Dakahlyia |
Broiler (800000) |
20 days |
Vaccinated (one time, H120 vaccine) |
Kidney damage with high morbidity and mortality rates |
|
IBV/Eg/Mans-6/12 |
Damietta |
Saso (20000) |
48 days |
Vaccinated (2 times, H120 vaccine) |
Severe respiratory manifestations |
IBV = Infectious bronchitis virus; Eg = Egypt; /Mans-1 to -6 code for the six different samples
Inoculation of Chicken Tracheal Organ Cell Cultures (TOCS)
TOCs were prepared according to Winter et al. (2008) and incubated at 37°C under rotation. Only TOCs with a ciliary activity of 100% were selected for comparing of the ciliostatic effect of isolated and reference viruses. The TOCs were infected by all isolates and reference strains using a concentration of 1x104 ffu/ml of each virus and incubated for one hour. The medium was changed daily with an observation and analysis of the ciliary movement at internals of 24, 48, 72, 96, and 120 hours post infection.
RNA Extraction and RT-PCR
The harvested allantoic fluids were centrifuged at 3000 rpm for 10 min. and the supernatants were used for viral RNA extraction using the QIAamp Viral RNA Mini Kit (Qiagen) according to manufacturer’s instructions. The RNA was eluted in 50 µl buffer. Subsequently, an one-step RT-PCR targeting the genetic information of the S1 subunit of the IBV S-protein was carried out for each of the six samples, using the Qiagen One Step RT-PCR Kit (Qiagen) in a total volume of 25 µl using 10 pmol of foward (S1Oligo5) and reverse (S6) primers. The used primers, leading to a product of 719 base pairs in length, were S1Oligo5: 5’- TGAAAACTGAACAAAAGACA-3’ (Kwon et al., 1993) and S6: 5’- ACATCWTGTGCGG -TGCCATT-3’ (Bochkov et al., 2006). The RT-PCR was performed in a thermal cycler with an initial denaturation at 95°C for 15 min., followed by 42 cycles, of denaturation for 30 seconds at 95°C, annealing for 1 min at 50°C, and extension for 1 min at 72°C. Afterwards, a final extension for 10 min at 72°C was performed. Next, the PCR products were isolated by standard agarose gel electrophoresis followed by gel purification, using the MinElute Gel Extraction Kit (Qiagen) according to manufacturer’s instruction. The purified products were sequenced by a commercial DNA sequencing service (LGC Genomics, Berlin) using the forward primer S1Oligo5.
Sequence Analysis
For analyzing the sequences of the spike glycoproteins (S1) from the six Egyptian IBV isolates, Mans-1 (IBV/Eg/Mans-1/12; GenBank: KF856872.1), Mans-2 (IBV/Eg/Mans-2/12; GenBank: KF856873.1), Mans-3 (IBV/Eg/Mans-3/12; GenBank: KF856874.1), Mans-4 (IBV/Eg/Mans-4/12; GenBank: KF856875.1), Mans-5 (IBV/Eg/Mans-5/12; GenBank: KF856876.1), and Mans-6 (IBV/Eg/Mans-6/12; GenBank: KF856871.1) on nucleotide and amino acid levels, the S1-protein sequences of the following avian IBV strains were used as references: Avian IBV strain QX (GenBank: AF193423), Italy 02 (GenBank: AJ457137.1), 4/91 (GenBank: JN192154.1), Beaudette (GenBank: DQ001334.1), Beaudette CK (GenBank: AJ311317.1), M41 (GenBank: DQ834384.1), H120 (GenBank: EU822341.1), Eg/CLEVB-1/IBV/012 (GenBank: JX173489.1), Egypt/F/03 (GenBank: DQ487085.1), IS/1494/06 (GenBank: EU780077.2), Sul/01/09 (GenBank: GQ281656.1), and IS/855 (GenBank: AY279533.1). The S1-protein sequences and the nucleotide identity (indicated in %) were calculated using the basic local alignment search tools BLASTp and BLASTn respectively, which are available at the webpage of the National Center for Biotechnology (NCBI; http://www.ncbi.nlm.nih.gov/). In both cases, the S1-protein sequences of all of the six isolates were individually aligned with each of the reference sequences (two sequence alignments). Additionally, a multi sequence alignment of all S1-protein sequences on amino acid level was performed using the ClustalW2 available under (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Nucleotides sequences were translated into amino acids sequences using the translate tool from the Bioinformatics Resource Portal of the Swiss Institute of Bioinformatics (SIB), which is available under (http://web.expasy.org/translate/).
Phylogenetic Analysis
To analyse the phylogenetic relationship of the six isolates and the aforementioned reference sequences, phylogenetic trees was constructed using the Phylogeny.fr tool (Dereeper et al., 2008), available under (http://phylogeny.lirmm.fr/phylo_cgi/index.cgi). The construction of the phylogentic tree is based on maximum likelihood by four implemented programs - MUSCLE 3.7 (alignment), Gblocks 0.91b (alignment refinement), PhyML 3.0 (phylogeny) and TreeDyn 198.3 (tree rendering) - and was performed on both the nucleotides and amino acids level.
RESULTS
Virus Propagation and Titration
The samples collected from the poultry farms in northern Egypt were inoculated via the allantoic cavity route in embryonated SPF chicken eggs. All isolates led to the development of characteristic IBV lesions as curling, dwarfing and stunting of the embryos. Two isolates, IBV/Eg/Mans-2 and -4, showed clear lesions of the embryos after the first passage increasing to four isolates after the second passage (IBV/Eg/Mans-1, -2, -4, and -5), while after the third passages, all isolates led to clear specific lesions (Table 2).
All samples, including the reference strains, were titrated in CEK cells and stained by IFT for detection of the viral cytopathic effect on the cell culture. All viruses showed plaques which appeared as foci, in CEK cell cultures. The viral titers ranged between 1x104-3.5x105 foci forming unit (ffu) /ml (Table 2).
Table 2: Result of serial passage in SPF egg inoculated by IBV isolates and the titration on CEK
|
Isolate |
Passage 1 |
Passage 2 |
Passage 3 |
Titer on CEK |
|
IBV/Eg/Mans-1/12 |
- |
+ |
+ |
1.5 x 104 |
|
IBV/Eg/Mans-2/12 |
+ |
+ |
+ |
2 x 105 |
|
IBV/Eg/Mans-3/12 |
- |
- |
+ |
1 x 104 |
|
IBV/Eg/Mans-4/12 |
+ |
+ |
+ |
3.5 x 105 |
|
IBV/Eg/Mans-5/12 |
- |
+/- |
+ |
1.2 x 104 |
|
IBV/Eg/Mans-6/12 |
- |
- |
+ |
1 x 105 |
|
Control |
- |
- |
- |
Curling and dwarfing of chicken embryos were assessed for the presence by (+) or absence by (-). The titration data indicate the mean of three independent experiments and calculated as ffu/ml.
Ciliostasis Analysis of the Isolates in TOCs
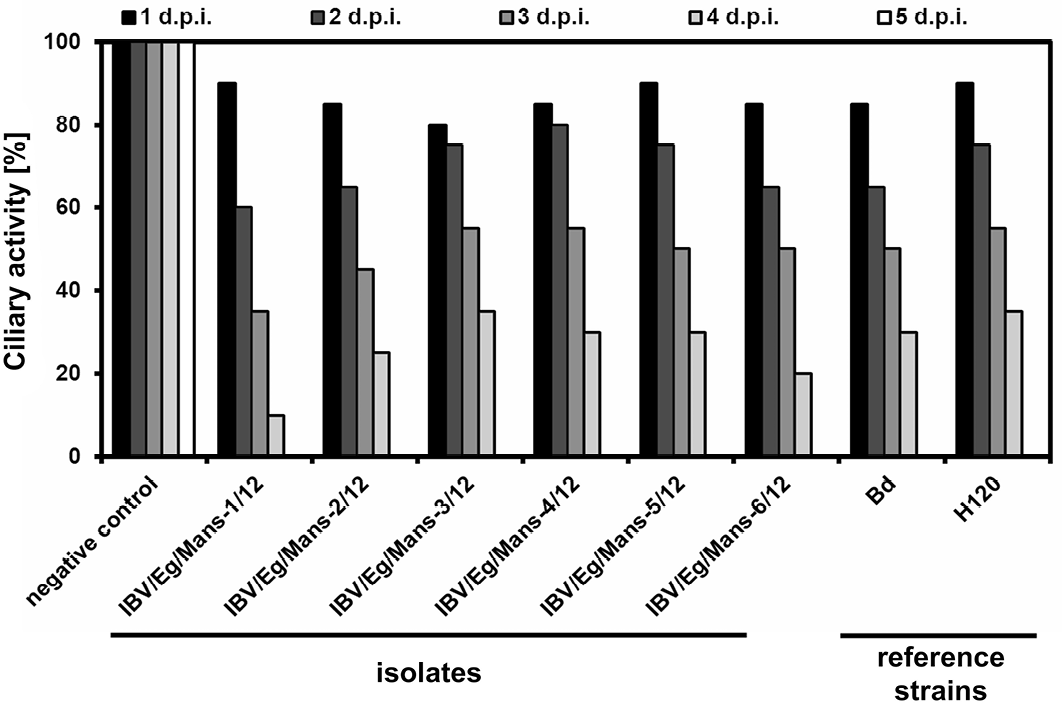
Inoculation of TOCs with an infectious dose of 1x104 ffu/ml for all isolates, Bd and H120 strains led to decrease the ciliary activity of TOCs stepwise in a similar fashion with only little variations, showing a complete ciliostasis at the fifth day post infection, while mock-treated TOCs kept their full ciliary activity at that time point (Figure 2).
Chicken TOCs inoculated by 1x104 ffu/ml of the IBV isolates (IBV/Eg/Mans-1, -2, -3, -4, -5, or -6/12) and the reference strains (Beaudette and H120); Mock-treated TOCs served as a negative control
Comparative Analysis of the Sequence Identity
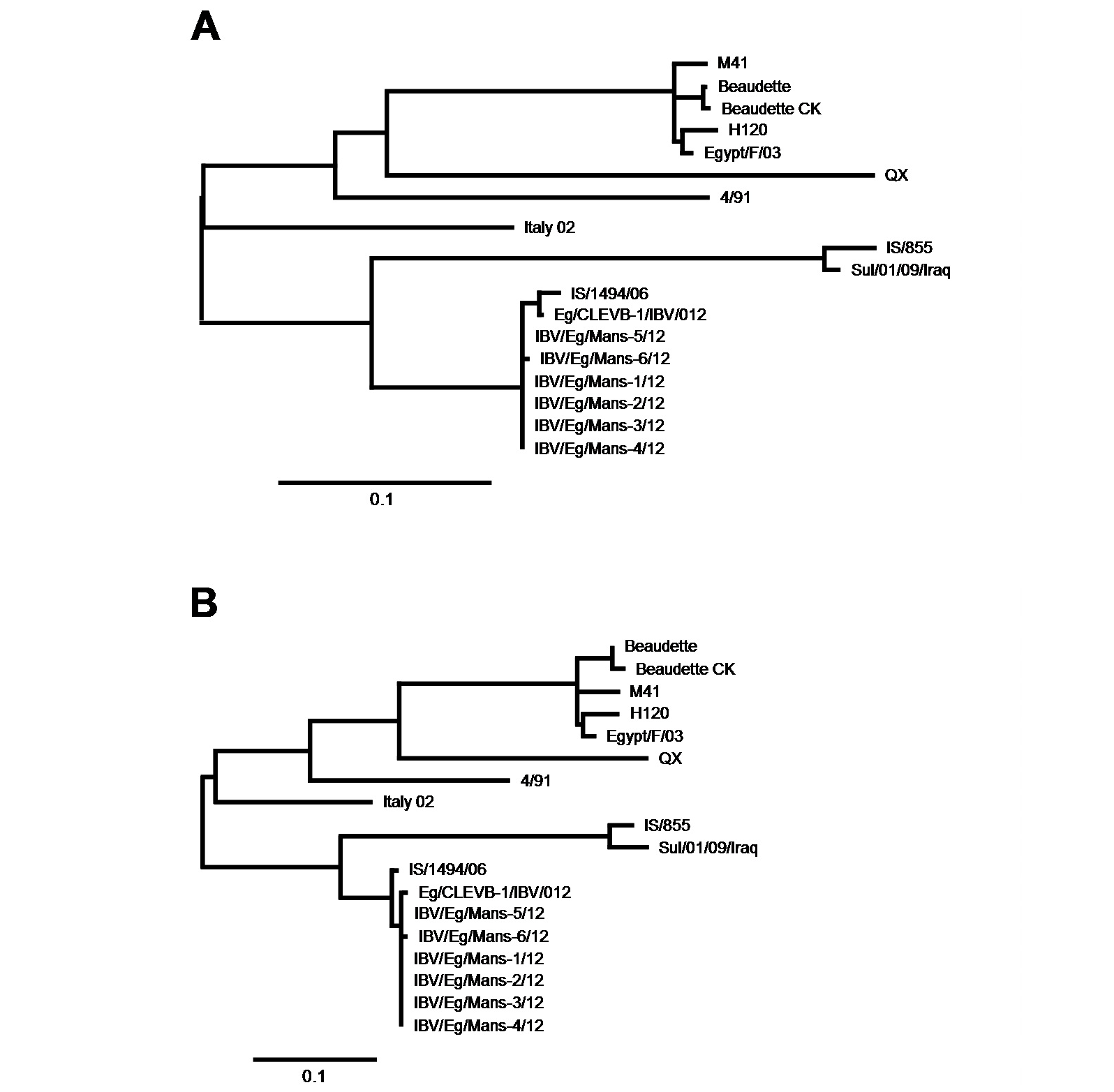
The genetic relationship comparison of the different reference and isolated viruses was determined using NCBI database (BLAST search) (Figure 3). The six isolates (IBV/Eg/Mans-1, -2, -3, -4, and -5) shared a sequence identity of 99 (IBV/Eg/Mans-6) to 100% on both neucleotides and amino acids levels (Figure 3 b and c). Compared to the reference strains, the highest sequence identity of the six isolates was obtained for the Egyptian Eg/CLEVB-1/IBV/012 and the Israeli IS/1494/06 strains with 99 and 98%, respectively. The sequence identity of the Egyptian isolates to the other reference strains ranged from 75% with QX and 4/91 strains to 81% with Sul/01/09/Iraq strain on nucleotide level and 67% with the M41 and H120 strains to 78% with IS/885 and Sul/01/09/Iraq strains on the amino acid level. Additionally, the conservation of amino acid residues of the six IBV isolates to the reference strains was compared by aligning the corresponding amino acid residues of the S1-proteins of the reference strains and the partial S1-protein sequence of the six isolates, using the ClustalW2 online tool. All amino acids residues within the partial S-protein sequence of the Egyptian isolates were represent in the S-protein sequences of some reference strains (Figure 4), especially the regions spanning from amino acid residues 28 - 50, 90 - 116, 164 - 183, and 191 - 200 showed a high conservation among all tested strains.
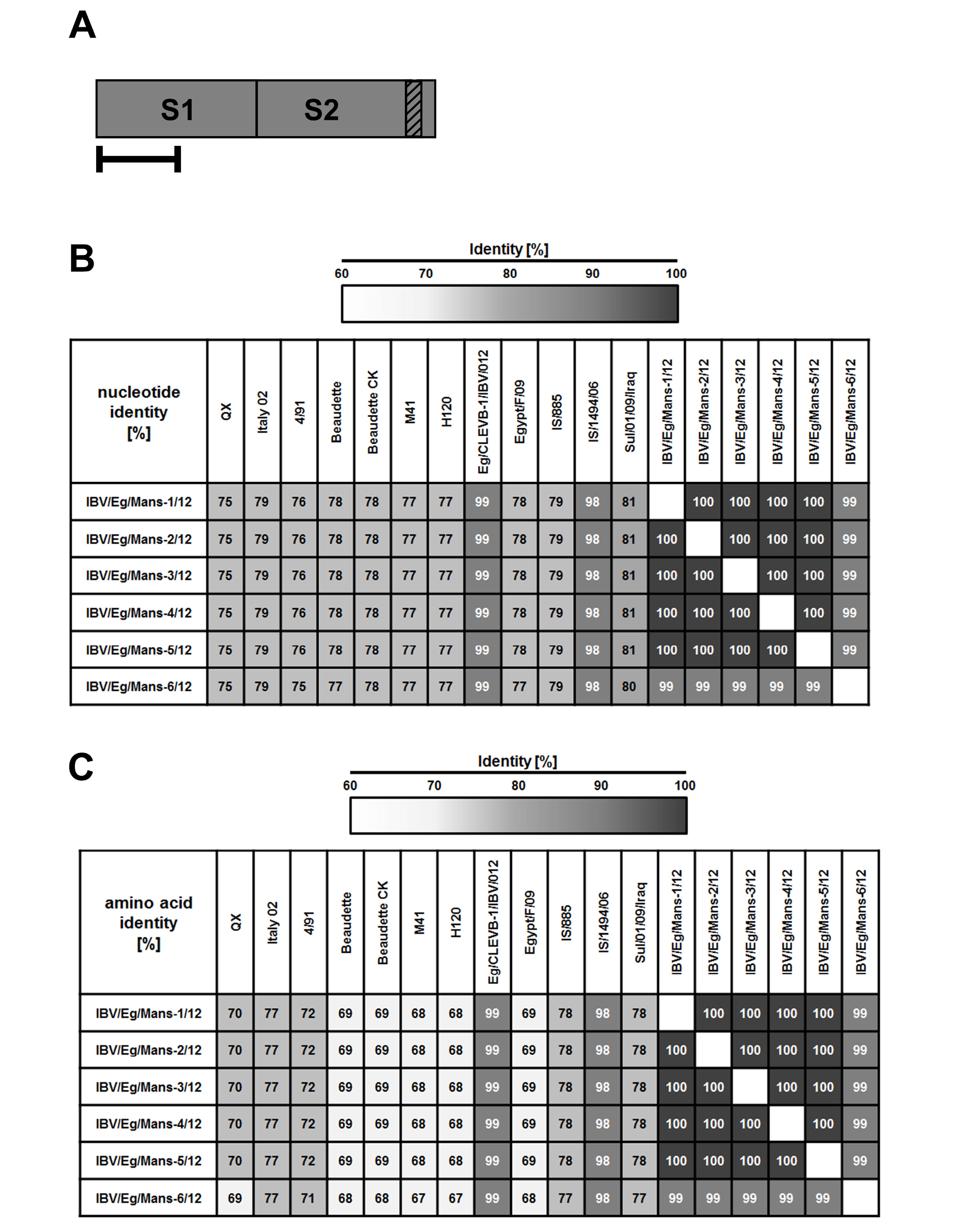
Figure 3: Sequence identities on the nucleotide and amino acid level of the Egyptian IBV isolates to reference strains based on their partial S1-protein sequences
By RT-PCR, partial S-protein sequences (A) were determined and used to calculate the total sequence identity on nucleotide (B) and amino acid level (C) for the Egyptian IBV isolates to selected reference strains. Numbers indicate the total sequence identity (in %).
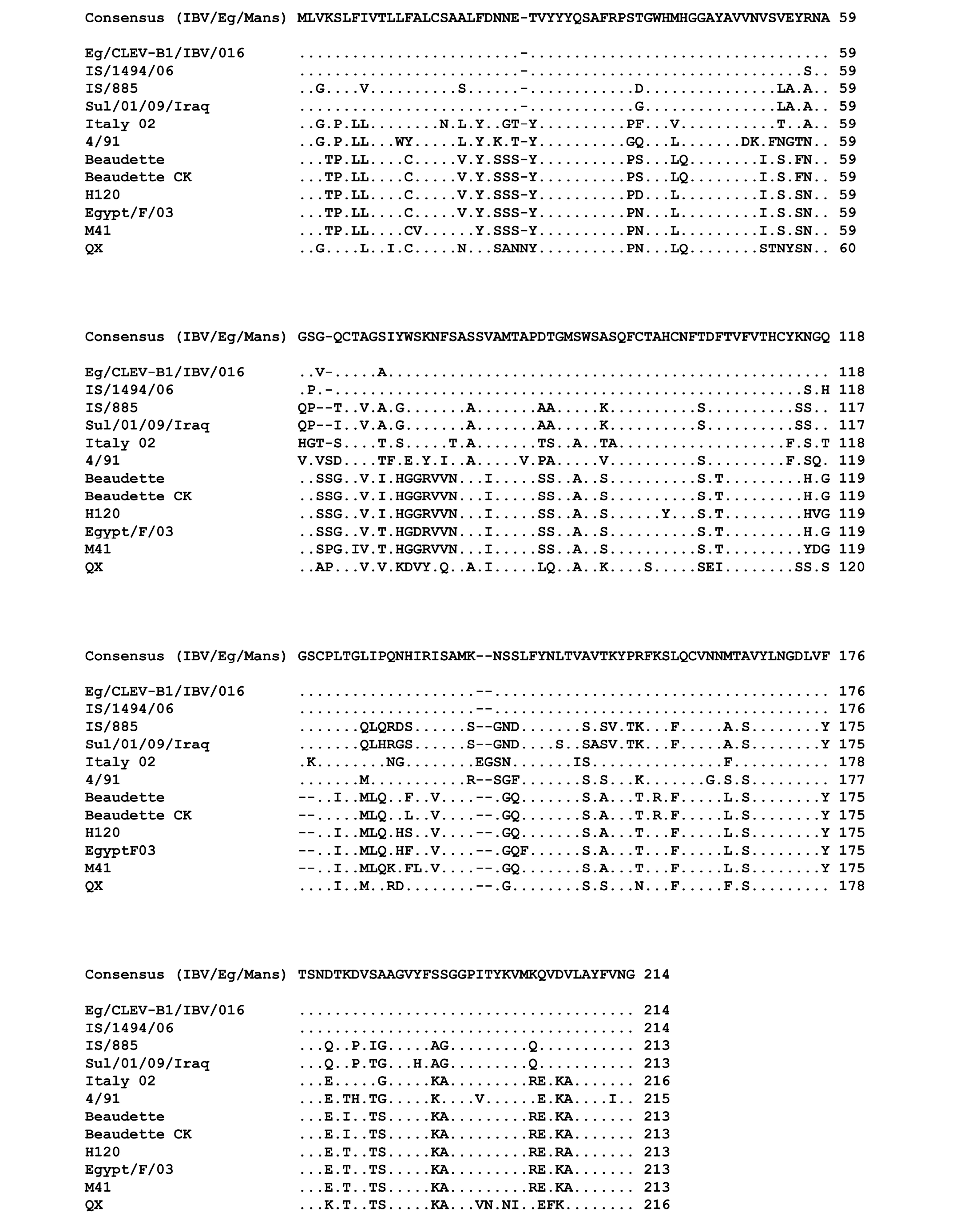
Figure 4: Multiple sequences alignment of the amino acid residues of the partial S-protein sequences for the IBV isolates and selected reference strains
Points indicate amino acid residues that are identical to the consensus sequence, while different amino acid residues are indicated by letters. Gaps which are a result of the alignment are indicated by a minus.
The partial S-protein sequences on nucleotide (A) and amino acid level (B) of the Egyptian IBV isolates. Scale bars indicate the number of nucleotide /amino acid substitutions per site.
Phylgenetic Analysis Based on the Partial S-Protein Sequences
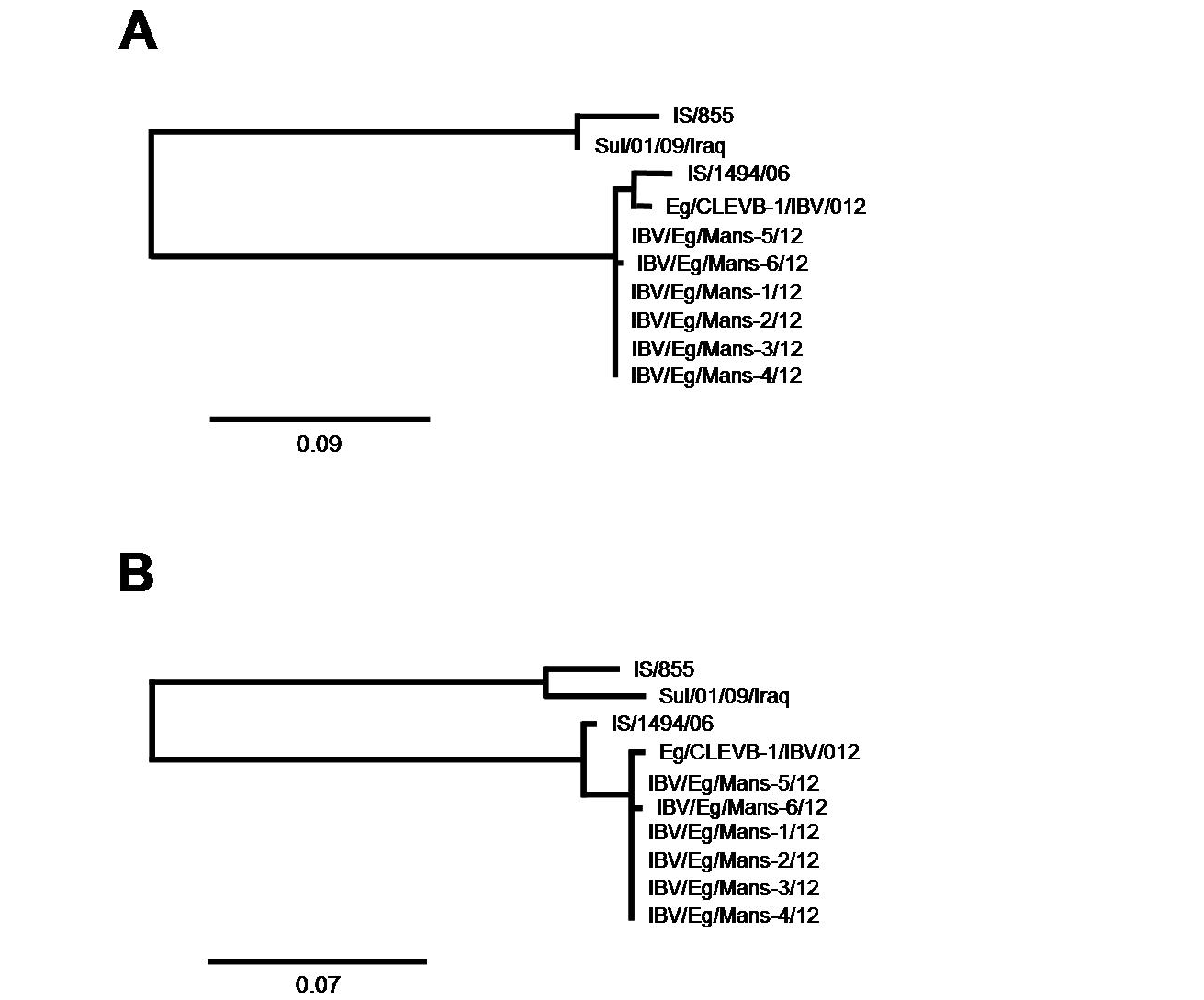
After having shown that the six IBV isolates shared the highest sequence identify on both nucleotides and amino acid levels with the Egyptian isolate Eg/CLEVB-1/IBV/012 and the Israeli isolate IS/1494/06, we analysed their phylogenetic relationship to the different reference strains based on the amino acids composition of the partial S1-protein sequences using Phylogeny.fr tool, that constructs phylogenetic trees based on maximum likelihood analyses. By construction of phylogenetic trees for the partial S1-protein sequences on nucleotides (Figure 5 and 6a) and amino acids level (Figure 5 and 6b), all the six Egyptian isolates form a distinct phylogenetic cluster together with the Egyptian Eg/CLEVB-1/IBV/012 and the Israeli IS/1494/06 strains (Figure 5). The Egyptian reference Eg/CLEVB-1/IBV/012 strain is closely related to the tested isolates than the Israeli reference IS/1494/06 strain (Figure 6).
The partial S-protein sequences on nucleotide (A) and amino acid level (B) of the IBV isolates. Scale bars indicate the number of nucleotide /amino acid substitutions per site.
DISCUSSION
IBV affects poultry farms causing great economic losses in poultry industry (Kwon et al., 2003; Webster et al., 2006; Nguyen et al., 2013). Although a live attenuated vaccine (H120) is used currently in Egypt, several poultry farms are still suffering from IBV infections. Thus, this study aimed to isolate the current circulating isolates of IBV in northern Egypt, characterize and compare them to other recently isolated strains in Egypt and neighbouring countries. The usage of embryonated chicken eggs for the diagnosis of IBV was considered as the method of choice for virus isolation (Cunningham 1970; Fabricant 1998). Characteristic curling and dwarfing were observed in the SPF eggs inoculated with the suspected samples after one to three passages. Although this diagnostic method has been established for quite a long time, it is still a preferable method for the propagation of IBV (Cook et al., 2012). Most IBV strains could be titrated in primary avian cells, especially of chicken embryo kidney cell culture, as they do not grow well in permanent cell lines. The Beaudette strain, however, is an exception, since it has an extended species tropism in cell culture (Otsuki et al., 1979). Recently, it has been suggested that the broader tropism may be related to the ability of this particulars virus to use heparin sulfates as an additional attachment factor (Mad et al., 2007), which might facilitate the entry into non-avian cell cultures. A virus titration by immunofluorescence analysis is commonly used not only for the titration of infectious viral particles but also to study the specificity and sensitivity of antibodies directed against IBV.
Primary tissue culture prepared from chicken trachea is used for not only studying the interaction between host species and pathogens but also comparing the pathological changes and the speed of virus replication of different IBV strains, i.e. QX strain is rapidly replicating virus causing complete ciliostasis of TOCs in the third day post infection comparable to the fifth day post infection for other relatively slowing replicating viruses as 4/91 and Beaudette strains (Lukert and Packer, 1972; Hu et al., 1975; Andrade et al., 1982; Winter et al., 2008; Abd El Rahman et al., 2009). In addition, TOCs can easily prepared, cultivated and since recent research is eager to establish alternative culture systems to reduce and/or replace infectious studies involving live animals. It is possible to evaluate the infection of TOCs by IBV via monitoring of the ciliary activity, as an infection by IBV results in a decrease of the ciliary movement, leading to complete ciliostasis a few days after the infection depending on the virulence of the virus (Cavanagh et al., 1999). In this study TOCs were used for compering the infectivity of the recently detected Egyptian IBV isolates as well as reference strains (Beaudette and H120). Both, the new isolates and the reference strains displayed a similar effect on the ciliary movement of TOCs, resulting in a complete ciliostasis at the fifth day post infection.
RT- PCR has been extensively used for the molecular detection of IBV in allantoic fluid extracts and has been described as a very efficient, sensitive and accurate method for the detection and identification of IBV types (Cavanagh et al., 1999). Additionally, RT-PCR facilitates the detection of new and/or emerging IBV variants different antigenic properties due to alterations in the S1 subunit of the IBV spike glycoprotein (S). Those molecular alterations of the IBV virus can enable it to escape the adaptive immune system, although it has been stimulated by routinely used vaccines (Gelb et al., 1991; Gelb et al., 1997). During the 2012 outbreaks, Abdel-Moneim and others could identify new genotypes of IBV in different locations in the middle and northern Egypt. Although we could not align our identified strains with the strains isolated by Abdel-Moneim and his group, due to differences in the amplicon size of the S1 gene, all identified genotypes in southern and northern Egypt are closely related to the Israeli strain (Abdel-Moneim et al., 2012).
The key features for emergence of new IBV variant strains among poultry and as a potential pandemic virus could be provided by genetic characterization of the circulating viruses in Egypt. Periodical molecular identification of the current circulating IBV genotypes in different parts of Egypt is a valuable tool for evaluating the molecular evolution and detecting the mechanism of new variants emergency in Egypt. Adopting a nationwide strategy for controlling IBV in Egypt is required by continuous monitoring of the new variant IBV strains and developing of suitable vaccines which need selective and represented virus for efficient controlling of the disease.
In conclusion, our study could describe the genetic characteristics of the IBV virus isolated from chicken in northern Egypt and provided evidences to further understand the mutation trend relevant of the IBV viruses. The increased morbidity and mortality, which associated with the poultry farms, might dente additional factors that affected the case severity and allowed the IBV virus to cause severe signs. All the IBV isolates are closely related to each other and have the closest relatedness, based on total sequence identity analysis and phylogenetic tree construction, with the Egyptian Eg/CLEVB-1/IBV/012 and the Israeli IS/1494/06 strain, while, the Italy 02 strain sharing the closest relatedness of the “well-known” strains (79 and 77% on nucleotide and amino acid levels, respectively).
ACKNOWLEGEMENT
Authors would like to thank the German academic exchange service (DAAD) financially support Sahar Abd El Rahman during Post. Doctorate scholarship to the Institute of Poultry Diseases, Faculty of Veterinary Medicine, Berlin Free University, Berlin, Germany.
CONFLICT OF INTEREST
The authors declare that they have no conflict of Interest.
REFERENCES