Advances in Animal and Veterinary Sciences
Research Article
Immunosuppressive Effects of Chicken Infectious Anaemia Virus on T Lymphocyte Populations Using Flow Cytometry and Hematological Parameters During Experimental Subclinical Infection in Chicks
Mohd Yaqoob Wani1*, Kuldeep Dhama2, Ruchi Tiwari3, Rajamani Barathidasan2, Yashpal Singh Malik4, Shambhu Dayal Singh2, Raj Kumar Singh5
1Immunology Section, Division of Veterinary Biotechnology, 2Avian Diseases Section, Division of Pathology, Indian Veterinary Research Institute, Izatnagar (U.P.)-243 122, India; 3Department of Microbiology, College of Veterinary Sciences and Animal Husbandry, Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu Chikitsa Vigyan Vishwa Vidyalaya Evam Go-Anusandhan Sansthan (DUVASU), Mathura (U.P.) -281 001, India; 4Division of Biological Standardization, Indian Veterinary Research Institute, Izatnagar (U.P.) -243 122, India; 5Indian Veterinary Research Institute, Izatnagar (U.P.), India.
Abstract | Chicken infectious anaemia virus (CIAV) is an economically important pathogen affecting poultry industry worldwide, and renders birds susceptible to secondary infections. The present study was designed to investigate the systemic immunosuppressive effects of CIAV on T lymphocytes bearing CD4 and CD8 receptors using flow cytometry and hematological parameters during experimental subclinical infection in chicks. Forty specific pathogen free (SPF) chicks of 6 weeks of age were randomly and equally divided into two groups. Infected group received 104.5 TCID50 of CIAV while control chicks were mock inoculated. All the chicks were regularly monitored for clinical signs, hematology parameters and CD4+ and CD8+ cell populations in spleen and blood. The experimental CIAV infection was confirmed by PCR testing of the tissue samples of experimental chicks, using VP2 gene specific primers, which yielded an expected amplicon size of 651 base pairs. The analysis of the hematological parameters showed significant decline in hematocrit value (PCV), total leukocyte count (TLC) and peripheral lymphocyte count (PLC) after 15 days post infection (dpi) but with no clinical signs of CIA. Flow cytometric analysis revealed that the percentage of CD4+CD8- and CD4-CD8+ T cells significantly decreased in the virus infected chicks at 15 dpi both in the spleen and blood (p<0.05). The results supported the fact that subclinical CIAV infections are also immunodepressive in nature; the virus replicates in primary lymphoid tissues and induces immunosuppression by decreasing both CD4+ and CD8+ T cells in chicks.
Keywords | Chicken infectious anaemia virus, Poultry, Immunosuppression, Flow cytometry, T lymphocytes, Haematology, Subclinical infection
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | January 04, 2015; Revised | February 06, 2015; Accepted | February 09, 2015; Published | February 12, 2015
*Correspondence | Mohd Yaqoob Wani, Indian Veterinary Research Institute, Izatnagar (U. P.), India; Email: mywani27@gmail.com
Citation | Wani MY, Dhama K, Tiwari R, Barathidasan R, Malik YS, Singh SD, Singh RK (2015). Immunosuppressive effects of chicken infectious anaemia virus on T lymphocyte populations using flow cytometry and hematological parameters during experimental subclinical infection in chicks. Adv. Anim. Vet. Sci. 3(3): 143-150.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.3.143.150
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Wani et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Chicken infectious anaemia virus (CIAV) is an important poultry pathogen, which causes chicken infectious anaemia (CIA), a clinical disease affecting young chickens of up to 3-4 weeks of age. The clinical disease is characterized by poor weight gain, aplastic anaemia, subcutaneous and muscular haemorrhages, generalized lymphoid atrophy and immunosuppression (McNulty et al., 1991; Adair, 2000; Miller and Schat, 2004; Schat, 2009; Todd, 2000; Dhama et al., 2008). However, subclinical infections characterized by production losses and vaccine associated complications can act as source of infection to other flocks (McNulty et al., 1991; Dhama et al., 2008). The virus infection increases the susceptibility of birds to secondary infections and due to its profound immunosuppressive effects, even vaccination failures may occur, altogether leading to huge economic losses to the poultry industry worldwide (Pope, 1991; Hagood et al., 2000; Dhama et al., 2008; Bhatt et al., 2011). CIAV is the smallest avian virus (23-25 nm size) belonging to the genus Gyrovirus of family Circoviridae. The viral genome consists of a circular ss-DNA of 2.3 kb having three partially overlapping major open reading frames (ORFs) which encodes for VP1, VP2 and VP3 proteins (Miller et al., 2005; Natesan et al., 2006). The VP1 (51 kD) acts as a major capsid protein and VP2 as a scaffold protein essential for virus assembly, while VP3 (apoptin) is important for the disease pathogenesis (Miller et al., 2005). The CIA has attained much importance due to the frequent outbreaks in commercial poultry farms in various countries and is considered as one of the emerging diseases with potential to act as severe threat to the poultry industry at global level (Ducatez et al., 2006; Ducatez et al., 2008; Kim et al., 2010; Oluwayelu, 2010; Bhatt et al., 2011; Snoeck et al., 2012; Nayabian and Mardani, 2013).
Pathogenesis of CIAV involves adsorption and penetration of the virus into hematopoietic and thymic precursor cells. The virus multiplies in the nucleus by a rolling circle model, thereby cause damage to stem cells in bone marrow and precursor T-lymphocytes in the thymus (Smyth et al., 1993; Dhama et al., 2008). Previous reports have shown that CIAV either destroys cells expressing CD4, CD8, and CT1 molecules on their surface or interferes with the expression of these molecules (Hu et al., 1993). Also, there are reports that mature T lymphocytes in the spleen are affected by CIAV infection (Adair et al., 1993). Few experimental studies indicated that CIAV has greater effect on CD8+ cells than CD4+ cells (Adair et al., 1993), while in few other studies no selective decrease in cytotoxic T lymphocytes (CTL) was detected (Hu et al., 1993). The age-related resistance develops to CIAV infection by antibody production by B cells; however CIAV can persist as a latent virus in spite of the presence of neutralizing antibodies (Miller and Schat, 2004). A recent molecular epidemiological study from India indicated CIAV positivity of 66.6% and 25%, respectively, in 3-7 week and 7-12 week age groups of chickens (Wani et al., 2013). Also, recent findings have shown that CIAV can cause characteristic histopathological changes and immunosuppressive effects involving various cytokines in the adult susceptible birds (Haridy et al., 2012a; Wani et al., 2014).
Therefore, the present study was designed to determine the immunosuppressive effects of CIAV on T lymphocyte populations bearing CD4 and CD8 receptors using flow cytometry and hematological parameters during experimental subclinical infection in chicks.
MATERIALS AND METHODS
Virus isolate
An Indian field isolate of CIAV (CIAV-E strain; GenBank accession no. AY583757), maintained in Avian Diseases Section, Division of Pathology, Indian Veterinary Research Institute (IVRI), Izatnagar was used during the present study.
Specific pathogen free (SPF) chicks
Embryonated specific pathogen free (SPF) chicken eggs (n=45) were obtained from M/S Venkateshwara Hatcheries Private Limited (VHL), Pune, Maharashtra, India and hatched in Hatchery Unit of Central Avian Research Institute, Izatnagar. The chicks were reared in Experimental Sheds of Avian Diseases Section, IVRI, under strict isolated conditions and good management practices. All the experimental procedures on animals were carried out according to the recommendations and approval of the Institute Animal Ethics Committee (IAEC) as per the guidelines set forth by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India.
Experimental design and sampling
Forty 6-week-old SPF chickens were randomly divided into infected (n=20) and control group (n=20). The chicks of the infected group were inoculated with 104.5 tissue culture infective dose (TCID50 / ml) of CIAV in 0.5 mL volume intramuscularly in thigh muscle; whereas control chicks received uninfected cell lysate as described previously (Natesan et al., 2006). All the chicks were regularly monitored for the clinical signs of the disease, and blood samples were collected at 0, 3, 7, 14 and 21 days post infection (dpi). For determining the effect of CIAV on CD4+ and CD8+ T cell populations, three birds from each group were sacrificed at 5, 15 and 25 dpi, and spleen and blood were collected for flow cytometry analysis.
Hematology
The hematological parameters assessed in this study included packed cell volume (PCV) (hematocrit values) using micro-hematocrit capillary tube method, total leukocyte count (TLC), peripheral lymphocyte count (PLC) and peripheral heterophil count (PHC) as per the standard procedures (Campbell, 1995). All these parameters were assessed using EDTA (2.0 mg mL-1) anti-coagulant-added blood from at least five birds in each group.
Flow cytometry
Sample preparation
Single cell suspension of lymphocytes from the blood and spleen were prepared for flow cytometric analysis. The single cell suspension of splenocytes were obtained by triturating and sieving the tissue through a nylon screen using Ca2+- and Mg2+-free phosphate buffered saline solution (PBS, pH 7.4) as described previously (Erf et al., 1998). Further, purification of lymphocytes was carried out by using Ficoll density gradient method (Histopaque 1077, Sigma Chemical Co., USA). The lymphocytes were then immunostained with mouse anti-chicken CD4+ FITC and CD8+ RPE conjugated antibodies (AbDSero Tech, U.K.) for flow cytometry. Briefly, the cells were enumerated by hemocytometer using trypan blue dye exclusion method and 106 cells were resuspended in 0.3 mL of FACS (fluorescent activated cell sorter) buffer (3% FCS, 0.09% NaN3 in PBS; pH 7.4) in 1.5 mL micro-centrifuge tube. For staining the cells, 10 µL of CD4+ FITC and 6 µL CD8+ RPE antibodies were added to each tube. After proper mixing, the cells were incubated for 1 hour at 4oC. The cells were washed with FACS buffer at 3,000 rpm for 5 minutes and finally resuspended in 200 µL of FACS buffer for acquisition.
Analysis of CD4+ and CD8+ T cells
For enumeration of CD4+ and CD8+ T cells, flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) was performed on FACS Calibur® instrument from Bioscience. The instrument setting was decided by using unstained cells and cells were stained with isotype control and each sample was acquired by taking 30,000 cell counts. The samples were analysed in “Cell quest” software of FACS Calibur (BD). As the monoclonals for CD4 and CD8 were conjugated to FITC and RPE labeled dyes, which are detected in FL2 channel and FL1 channels, respectively, histograms and dot plots were drawn with FL 1 and FL 2 channel. The positive cell counts were displayed in histogram stat and dot plots.
Virus detection by PCR
The technique of polymerase chain reaction (PCR) was used for confirmation of the establishment of CIAV infection in experimentally infected chicks using whole DNA isolated from host tissues (thymus, liver and spleen). In brief, the DNA was isolated from the pooled tissue samples from sacrificed birds at 15 dpi, using DNeasy® Blood and Tissue Kit (QIAGEN, Germany) following manufacturer’s instructions. The PCR amplification of VP2 was performed using gene-specific forward (5’ atgcacgggaacggcggac 3’) and reverse (5’ tcacactatacgtaccgggg 3’) primers (Basaraddi et al., 2013). The known CIAV positive and negative DNA samples were used as standard positive control and negative control, respectively.
Statistical analysis
All the data are presented as mean ± MSD and the experimental groups were compared by ANOVA followed by a post hoc Tukey’s test using SPSS v.16.0 statistical software. The values with p<0.05 were considered statistically significant.
RESULTS
Hematological changes
The hematological parameters including haematocrit value (HV), total leukocyte count (TLC), peripheral lymphocyte count (PLC) and peripheral heterophil count (PHC) assessed in heparinized blood collected at 3, 7, 14 and 21 dpi of CIAV infection showed significant decrease in HV and TLC values. However, no apparent clinical signs of CIA were observed in the virus infected group of chicks. The HV in normal uninfected control chicks and in experimental group before the virus infection was in the range between 30.08 ± 2.87 and 33.30 ± 3.71, respectively. The HV reduced to 25.52 ± 4.12 and 28.21 ± 3.02 in infected chicks on 14 and 21 dpi, respectively. At 14 dpi, TLC was reduced to 12.67 ± 1.26 x 103/mm3 compared to 56.71 ± 7.25 x 103/mm3 in uninfected birds at 0 dpi. Similarly, significant differences were also observed in PLC (%) among the infected chicks and control groups. However, no significant differences were observed in PHC in the infected and control group chicks (Table 1).
Table 1: Effect of CIAV inoculation on packed cell volume (PCV), total leukocyte count (TLC), peripheral lymphocyte count (PLC) and peripheral heterophil count (PHC) in experimentally infected chicks.
|
Parameters |
Group |
CIAV Post inoculation day |
|||
|
3 |
7 |
14 |
21 |
||
|
|
|||||
|
PCV (%) |
Control |
33.30 ± 3.71 |
32.17±3.60 |
30.08 ± 2.87B |
31.08 ± 3.30 |
|
Infected |
32.77 ± 1.76a |
30.12±3.61b |
25.52 ±4.12Ac |
28.21 ± 3.02b |
|
|
TLC (x103/mm3) |
Control |
16.72 ± 3.76 |
16.13 ± 2.06 |
17.56 ± 2.48A |
17.68 ± 2.96C |
|
Infected |
17.02 ± 2.87a |
14.51 ± 1.87 b |
12.67 ± 1.26Bc |
15.64 ± 2.88b |
|
|
PLC (%) |
Control |
55.34 ± 5.32 |
56.76 ± 4.29 |
56.71 ± 7.25 |
56.71 ± 6.86 |
|
Infected |
57.89 ± 7.36a |
54.63 ± 6.56a |
48.76 ± 3.69Bb |
49.80 ± 5.76b |
|
|
PHC (%) |
Control |
23.53 ± 2.43 |
25.70 ± 3.18 |
23.98 ± 2.44 |
25.63 ± 3.74 |
|
Infected |
24.76± 3.67 |
23.67 ± 2.55 |
26.07 ± 2.65 |
27.83 ± 4.81 |
|
The values (Mean ± SD) having at least one common superscript (Capital letters in columns and small letters in rows) do not differ significantly (P <0.05) for a given parameter.
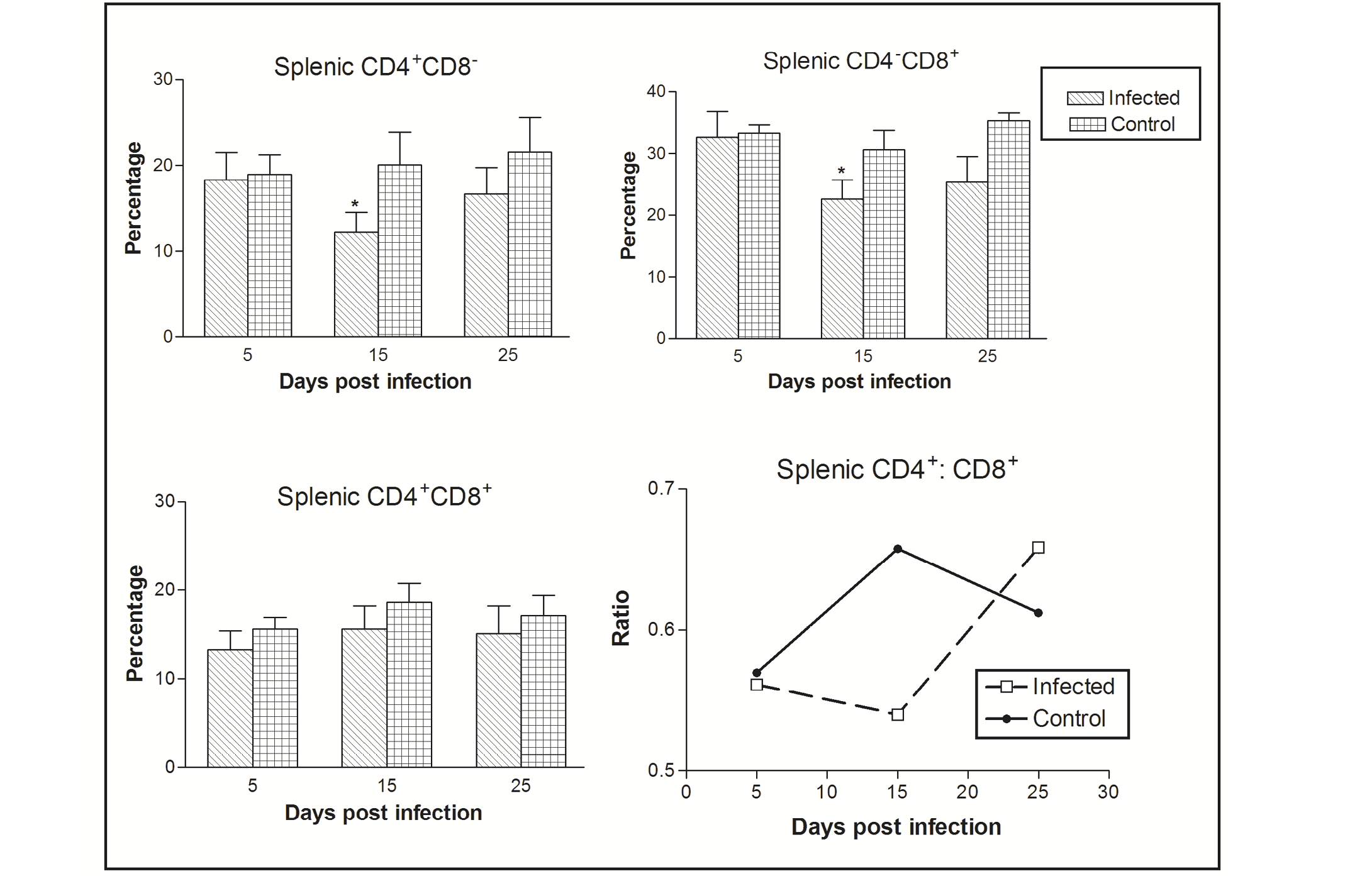
Figure 1: Effect of CIAV infection on splenic T lymphocytes in 6-week-old infected chicks at various post infection days. Values are represented as Mean ± SD, * p <0.05
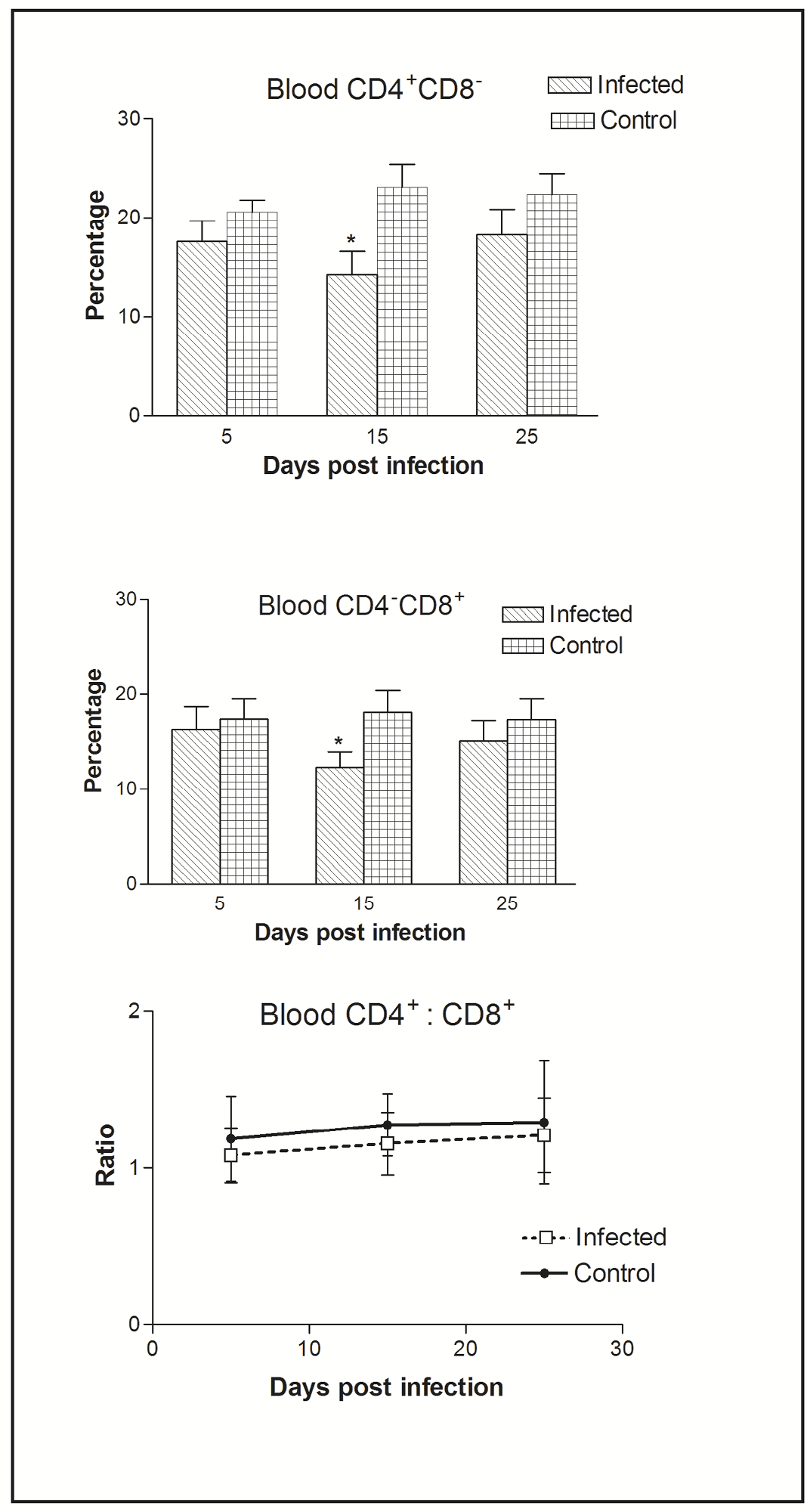
Figure 2: Effect of CIAV infection on T lymphocytes in blood at various post infection days. Values are represented as Mean ± SD, * p <0.05
CD4 and CD8 T cell population analysis
In the spleen, the percentage of CD4-CD8+ cells was greater as compared CD4+CD8- to CD4+CD8+ cells. The CIAV infection was found to decrease all the three T cell population types. Significant differences were observed in CD4-CD8+ and CD4+CD8- cells at 15 dpi. The ratio of CD4+CD8- : CD4-CD8+ cells was always less than one in both the infected as well as control groups, however it increased in chicks of the virus infected group at 25 dpi (Figure 1).
In blood, flow cytometric analysis of cells indicated the presence of higher percentage of CD4+CD8- compared to CD4-CD8+ cells. Thus, CIAV infection significantly decreased the peripheral CD4+CD8- and CD4-CD8+ cells. However, the CD4+CD8-: CD4-CD8+ ratio was always higher (>1) compared to that of splenic ratio (Figure 2).
Virus detection by PCR
The CIAV VP2 gene specific PCR, performed for the detection of the CIAV in the pooled tissue samples (liver, thymus and spleen) in both the infected and control group of chicks, showed a distinct amplicon of 651 bp in 1% agarose gel electrophoresis only with the tissues of the virus infected chicks (Figure 3). No amplification was observed in tissues obtained from uninfected control group chicks.
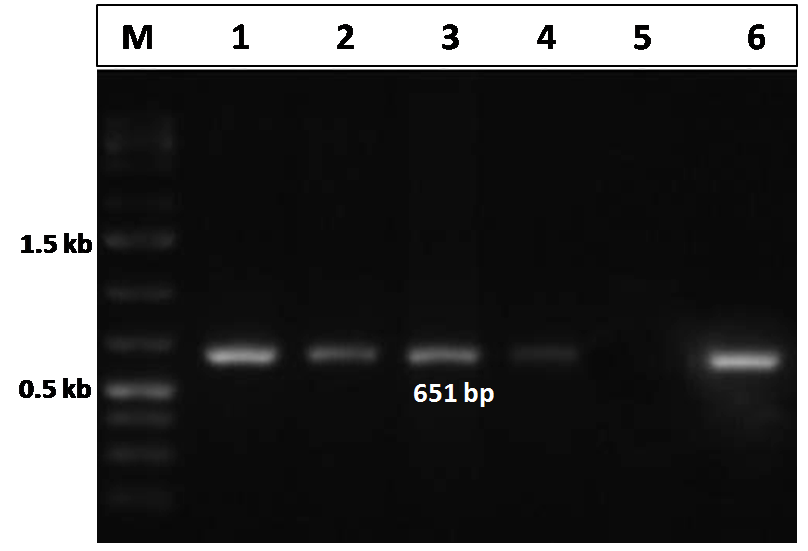
Figure 3: Confirmation of CIAV infection by PCR amplification of the VP2 gene of CIAV in the experimentally infected birds
Lane 1, 2, 3, 4: tissues samples from chicks of CIAV infected group; Lane 5: Negative control; Lane 6: CIAV positive control; Lane M: 1Kb DNA ladder.
DISCUSSION
In the present study, distinctive haematological changes were observed in the CIAV infected chicks. A significant (P<0.05) decline in the haemoglobin (Hb), PCV and TEC was observed in the CIAV infected group as compared to the healthy control chicks, although clinical signs of CIA were not apparent in the infected group chicks. The PCV was significantly decreased in comparison to control chicks at 15 dpi, however PCV at 25 dpi showed hint of recovery which may be due to regeneration of haematopoietic precursor cells and recovery from the infection by the involvement of humoral immune responses (Adair, 2000; Dhama et al., 2008). The leukocytic lineages also showed a significant (P<0.05) decline in TLC and PLC values in the chicks of virus infected group as compared to control. All these changes were in agreement with the previous reports (Bhatt et al., 2013; Latheef et al., 2013). Recent findings have shown that in the adult susceptible birds, CIAV replicates at high concentration in the thymus, and causes characteristic histopathological changes in the thymus, spleen, bursa of Fabricius, proventriculus and caecal tonsils (Kaffashi et al., 2006; Haridy et al., 2012a; Wani et al., 2014). Infections with CIAV increases the susceptibility of birds to secondary (bacterial/viral) infections, depresses vaccinal immunity, aggravates residual pathogenicity of attenuated virus and vaccine strains leading to vaccination failures and various disease outbreaks (Pope, 1991; Adair et al., 1993; Todd, 2000; Dhama et al., 2003; Dhama et al., 2008). Although the clinical disease generally occurs during first 2-3 weeks of age, subclinical infections frequently occur in adult birds. The virus causes suppression of hematopoietic precursor cell proliferation and differentiation and, thereby leads to transient destruction of erythroblastoid and granuloblastoid cell lineages in bone marrow. This is characterized by drastic reduction in the production of mature red blood cells (erythropoiesis) and myelopoiesis leading to hypoplasia, anaemia and panleukopenia (McNulty, 1991; Pope, 1991; Dhama et al., 2008).
The primary target of CIAV includes the T lymphocytes while B lymphocytes are resistant to the virus penetration and replication. In the present study, the T lymphocytes were analysed in the spleen and peripheral blood using CD4 and CD8 cell specific monoclonal antibodies. The results showed that even in adult infected birds the CIAV replication decreased the cells bearing CD4 and CD8 receptors at all the post infection time intervals. The effects were more pronounced and highly significant at 15 dpi (p<0.05). In spleen, the ratio of CD4+CD8-:CD4-CD8+ cells was always less than one (<1), both in the infected and control groups, however it was increased in infected chicks at 25 dpi indicating the recovery and clearance of the CIAV as indicated by simultaneous recovery in haematological parameters. Similarly, flow cytometric analysis of peripheral blood lymphocytes indicated significant decrease of CD4+CD8- and CD4-CD8+ cells in the chicks of CIAV infected group. Such destructive effects on lymphoid organs in adult infected chicks were observed immunohistochemically by other workers during subclinical infection (Smyth et al., 2006; Haridy et al., 2012a). The blood CD4+CD8-:CD4-CD8+ ratio was low (1-1.2: 1) as compared to 1.2–3.25 ratio which may be due to SPF nature of the chicks. It is suggested that in SPF chicks the immune system is not stimulated properly as compared to commercially raised chicks which may be responsible for less number of T helper cells. Even in adult birds, experimental CIAV infection was found to cause significant decrease in thymic to body weight ratio and reduce the cytokine expression levels of important cytokines responsible for mounting effective immune responses and T cell development (Wani et al., 2014). Further, to confirm that the pathological changes were indeed induced by CIAV, PCR was used to detect the presence of virus in the infected chicks. The detection of CIAV by PCR has advantages like of being easy, economical, convenient and rapid as compared to the virus isolation or serological and immunohistochemical diagnostic methods (Kataria et al., 2005; Dhama et al., 2008; Oluwayelu, 2010; Wani et al., 2013). The VP2 gene amplification produced the expected product size of 651 bp, which was in conformity with earlier reports (Basaraddi et al., 2013).
It is important to mention here that by 3-6 weeks of age period the maternally derived antibodies vanish and makes chicks highly susceptible to horizontally transmitted CIAV infection (Dhama et al., 2008). Also, the co-infection with immunosuppressive pathogens like Marek’s disease virus, infectious bursal disease virus increases the severity of CIAV-associated complications to the growing and susceptible adult chicks (Haridy et al., 2012a; Haridy et al., 2012b). Although clinical disease does not occur in adult birds but such infections lead to decrease in body weight gain, contamination of pathogen-free eggs and vaccination failures; besides acting as source of infection to other susceptible birds. CIAV-infected birds show profound immunosuppression during concurrent infection with other viruses such as fowl adenovirus, reoviruses and Newcastle disease virus, leading to synergistic effects of both agents and increase in the susceptibility age period (Pope, 1991; Todd, 2000; Dhama et al., 2008).
In conclusion, the findings of the present study supported that CIAV can replicate and induce immunosuppression in the susceptible chicks during subclinical infection stages as indicated by the reduction in haematological parameters and in both of the CD4+ and CD8+ T lymphocyte populations at 15 dpi in the virus infected birds. The systemic effects of CIAV on T lymphocytes bearing CD4 and CD8 receptors during subclinical infection as analysed by flow cytometry indicated non-specific tropism of the virus for these cells. The replicative nature, carrier stages and immunosuppressive potential of the virus both in clinical and subclinical infection warrants the effective implementation of rapid diagnostic and appropriate control measures so as to prevent the production losses caused by this economically important pathogen of poultry, particularly the developing countries like India.
ACKNOWLEDGEMENTS
The authors are highly thankful to DBT and ICAR-NAE Projects, Delhi for strengthening facilities for CIAV research at IVRI, Izatnagar.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES




