Advances in Animal and Veterinary Sciences
Short Communication
Detection of Bordetella bronchiseptica in Serum of Apparently Healthy and Clinically Sick Pet Dogs
Polem Lenin Singh1, Bhoj Raj Singh1*, Monika Bhardwaj2, Prassanvadhana2, Dharmendra Kumar Sinha1, Nongthombam Boby3, Ravi Kant Agrawal4, Abhijit M Pawde5
1Division of Epidemiology, 2Division of Bacteriology and Mycology, 3Division of Animal Biotechnology, 4Division of Livestock Products Technology, 5Referral Veterinary Polyclinic, Indian Veterinary Research Institute (IVRI), Izatnagar-243122, Bareilly, India.
Abstract | DNA extracted from serum samples collected from 114 apparently healthy and 71 clinically sick pet dogs of different age, sex, breed and place was used as templet for detection of Bordetella infection through genus specific PCR (gPCR) targeting alc gene and species specific PCR (sPCR) targeting fim gene. Bordetella DNA could be detected in serum of 7 apparently healthy and 7 sick dogs both with gPCR and sPCR. Clinical status (p= 0.35), age (p= 0.31) and sex (p= 0.72) had no significant association with detection of Bordetella DNA in serum samples. Of the 14 samples positive 12 (85.7%) were from Kerala and two (14.3%) from Manipur dogs. Sequencing of the gel purified gPCR product targeting alc gene revealed 95% homology with alc gene sequence of several species of Bordetella and sequencing of the gel purified sPCR product targeting fim gene revealed 100% identity with Bordetella bronchiseptica fim gene. The study suggested that B. bronchiseptica might be the most common bordetellae affecting pet dogs irrespective of clinical illness. The study concluded that PCR with serum extracted DNA template can be an option for rapid detection of Bordetella infection in pet dogs.
Keywords | Bordetellosis, Bordetella bronchiseptica, PCR, Serum-PCR, dogs, Kennel cough
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 19, 2014; Revised | January 13, 2015; Accepted | January 14, 2015; Published | January 23, 2015
*Correspondence | Bhoj Raj Singh, Indian Veterinary Research Institute, Izatnagar, Bareilly, India; Email: brs1762@gmail.com
Citation | Singh PL, Singh BR, Bhardwaj M, Prassanvadhana, Sinha DK, Boby N, Agrawa RK, Pawde AM (2015). Detection of Bordetella bronchiseptica in serum of apparently healthy and clinically sick pet dogs. Adv. Anim. Vet. Sci. 3(2): 123-127.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.2.123.127
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Singh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Kennel cough is a multi-etiological disease and Bordetella bronchiseptica is the main etiology in dogs (Keil and Fenwick, 1998). Bordetella affects dogs of all the age groups (Appel et al., 1987; Ford, 2006) and has a zoonotic aspect too (Woolfrey et al., 1991) affecting immunocompromised (Bauwens et al., 1992) as well as healthy humans (Lo et al., 2001). Many conventional bacteriological methods, immunological (agglutination, microagglutination and enzyme linked immunosobant assay) and molecular tools (polymerase chain reaction, PCR; real time PCR) have been used to detect Bordetella infection in animals. However, diagnosis of bordetellosis is a problem because of slow growing and fastidious nature of the pathogen and contaminants in the clinical samples (Kumar et al., 2014; Bhardwaj et al., 2013a, b; Bhardwaj, 2013; Rapuntean and Rapuntean, 2010; Sacco et al., 2000).
Attempts have been made to identify circulating DNA in blood and or serum where isolation and identification of the pathogen from clinical samples is difficult even after using the best methods (Bougnoux et al., 1999; Kawamura et al., 1999; Murdoch et al., 1996; Zerva et al., 2001) or when etiology is obscure (De-Madaria et al., 2005). For diagnosis of brucellosis (Zerva et al., 2001) and candidiasis (Bougnoux et al., 1999) in human DNA template extracted from serum has proved more sensitive and specific template than extracted from whole blood samples, probably due to co-purification of PCR inhibitory substances with pathogen DNA (Akane et al., 1994). The well proven diagnostic method for infections in human has little known utility of the serum extracted pathogen DNA in diagnosis of infections in animals including for diagnosis of Bordetella infection. As collection of deep nasal swabs required for diagnosis of bordetellosis is difficult to collect even from well-trained dog and thus the diagnosis. Antibody detection methods though simple but lacks specificity thus have no diagnostic utility (Kumar et al., 2014; Bhardwaj et al., 2013a, b). This study was undertaken to explore the utility of serum samples for detection of antigen rather than antibodies for specific diagnosis of bordetellosis through identifying circulating Bordetella bronchiseptica DNA in the serum samples of apparently healthy and clinically sick pet dogs.
Out of 185 serum samples, 14 (7.6%) samples were positive for both genus specific amplicon (gPCR) and species specific PCR targeting alc (Figure 1) and fim (Figure 2) gene, respectively (Table 2). In earlier studies on the same lot of dogs using DNA template extracted from deep nasal swabs only four dogs were detected positive for B. bronchiseptica infection and B. bronchiseptica could be isolated from only one sample without showing any correlation between detection of B. bronchiseptica and kennel cough in dogs (Bhardwaj, 2013). All the five dogs positive for Bordetella in nasal swabs were also positive for PCR using DNA templet extracted from respective serum samples indicating 100% sensitivity of the serum PCR method. Probability of detection of B. bronchiseptica was significantly (p= 0.016) higher in serum extracted DNA than detection of Bordetella DNA in nasal swabs of dogs (Bhardwaj, 2013). Findings indicated the superiority of serum samples for PCR based detection of Bordetella bronchiseptica infection over nasal swabs.
Detection of B. bronchiseptica by PCR in serum samples had no significant (p= 0.35) association with health status either in male (p= 0.75) or female (p= 0.34) dogs. Further there appeared to be no significant (p= 0.72) association of sex of dogs with probability of detection of Bordetella DNA in serum samples. In earlier studies too detection of Bordetella in nasal swabs of dogs could not be associated with kennel cough however, detection of B. bronchiseptica in bitches was significantly higher (p= 0.03) than in male dogs and sex has been suggested an important predisposing determinant associated with the infection (Bhardwaj et al., 2013a). The observations indicated that we may reach on different conclusions depending on sensitivity of methods used in the study.
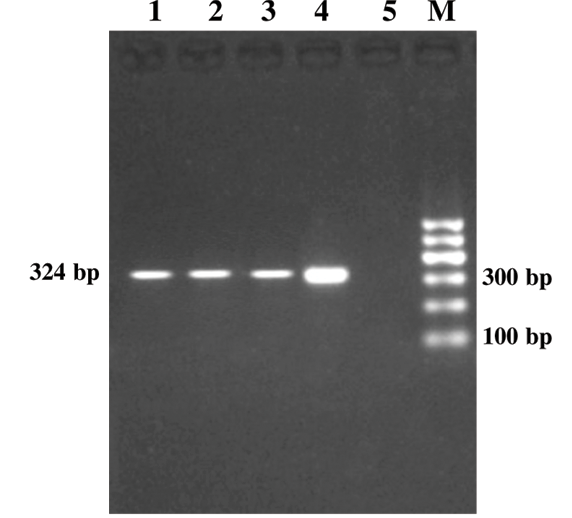
Figure 1: Genus Specific PCR (alc gene)
Lanes 1-3:Study samples; Lane 4: Positive control; Lane 5: Negative control; Lane M: 100bp DNA ladder
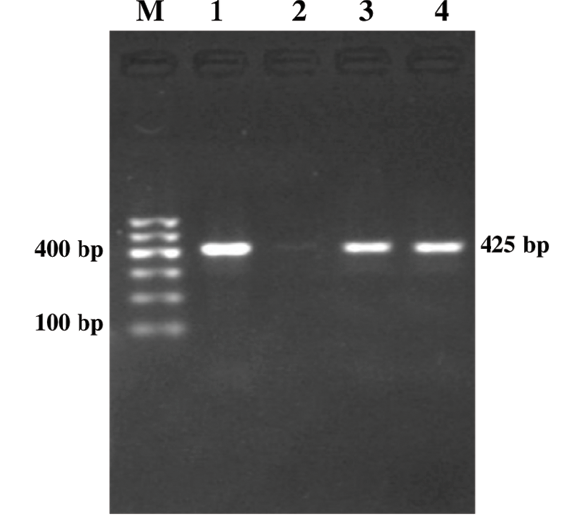
Figure 2: Genus Specific PCR (fim gene)
Lane M: 100bp DNA ladder; Lane 1: Positive control; Lane 2: Negative control; Lanes 3-4: Study samples
Table 1: Primers for Bordetella bronchiseptica detection
|
Name of primers |
Sequence 5' → 3' |
Target gene and product length (bp) |
References |
|
B688Bbalc-F |
ACCAACCGCATTTATTCCTACTA |
alc, 324 |
Bharadwaj, 2013 |
|
B1012Bbalc-R |
GGCCCTGGAGTTCGTATTTATG |
||
|
425BBfim-1 F |
TGAACAATGGCGTGAAAGC |
fim, 425 |
Xin et al., 2008 |
|
425BBfim-2 R |
TCGATAGTAGGACGGGAGGAT |
Note: (F), Forward primer; (R), Reverse primer
Table 2: Detection of Boretella DNA in serum of pet dogs in relation to their sex and health status
|
Sex |
Health status |
Total samples tested |
Positive for gPCR |
Positive for sPCR |
|
Male |
Apparently healthy |
77 |
5 |
5 |
|
Sick |
37 |
3 |
3 |
|
|
Female |
Apparently healthy |
37 |
2 |
2 |
|
Sick |
34 |
4 |
4 |
|
|
Total |
185 |
14 |
14 |
The association of B. bronchiseptica infection with kennel cough is well documented and reported frequently at several places (Appel et al., 1987; Keil and Fenwick, 1998; Ford, 2006). However, in the present study probability of detection of B. bronchiseptica DNA in serum of sick dogs (9.86%) was only insignificantly higher (p= 0.35) than detection of the pathogen from apparently healthy dogs (6.14%). The findings are in concurrence to earlier studies in India on bordetellosis and kennel cough in dogs (Bhardwaj et al., 2013a, b). The study also indicated that just colonization of B. bronchiseptica in dog nares or detection of pathogen’s DNA in serum may not be sufficient for diagnosis of bordetellosis and other factors might be important for precipitation of clinical disease in dogs.
All 14 positive samples with genus specific (alc gene) PCR were also positive for species specific PCR targeting fim gene in the study indicating that only B. bronchiseptica was able to invade the dog tissues. However, earlier study on nasal swabs indicated that although B. bronchiseptica was the major bordetellae colonizing dog nares other bodetellae might be present in nasal swabs of dogs (Bhardwaj et al., 2013a). In earlier studies on dogs (Bhardwaj, 2013, Bhardwaj et al., 2013a) out of total 7 nasal swabs positive to gPCR only four samples shown fim and fla genes amplification indicating that other species of Bordetella might be colonizing nasal mucosa of dogs.
Sequencing result of alc and fim gene PCR products revealed that the PCR performed in the study was true and specific in detecting only Bordetella.Sequences alignment of genus specific alc gene sequence amplified in the study had 95% identity with sequences of several species of Bordetella available in database and species specific fim gene sequence matched 100% with B. bronchiseptica sequences in database. The study concluded that PCR with serum extracted DNA might be an option for diagnosis of Bordetella infection in dog however there seems to be no association between kennel cough and Bordetella detection in dogs in India.
The study concluded that DNA extracted from serum samples can be used in molecular detection of Bordetella DNA for diagnosis of Bordetella infection in pet dogs. Clinical status and sex had no significant association with detection of Bordetella DNA in dog’s serum samples. Genus specific (alc gene) and species specific (fim genes) PCR results revealed that B. bronchiseptica is probably the most common invading Bordetella species affecting dogs.
Samples (deep nasal swabs and serum) were collected from 114 apparently (77 male and 37 female) healthy and 71 clinically sick (37 male and 34 female) pet dogs. Template DNA was extracted from serum samples using QIAmp DNA Mini kit (Qiagen, Germany) following the protocol recommended. From nasal swabs DNa was extracted after pre-enrichment step (Bhardwaj et al., 2013a) using the same kit. The concentration and purity of the DNA extracted from the serum as well as nasal swab samples were measured by Nano drop (Thermo Scientific, USA) and DNA samples with sufficient amount of concentration and purity indicated by 260/280 showing reading 1.6 to 1.8 were used as template for PCR reaction. The DNA extracted from the serum and nasal swabs was stored at -20°C till used in PCR. The amplification of genus specific and species specific primers were carried out using custom synthesized (Eurofin Pvt. Ltd., India) primers (Table 1). PCR reaction was optimized in 25µl reaction volume using 5 µl of DNA template, 1 µl each of 10 pMol forward and reverse primers, 12.5 µl of master mix and 5.5 µl of nuclease free water. The amplification of PCR was carried out in a thermal cycler (Eppendorf, Germany) with an initial denaturation at 95oC for 10 min, 35 cycles of denaturation at 94oC for 30 s, annealing at 53oC for 30 s, extension at 72oC for 45 s, followed by final extension step at 72oC for 7 min. Amplicons of 324 bp for genus specific PCR targeting alc gene and amplicon of 425 bp for species specific PCR targeting fim gene were analysed under UV-gel documentation system (Alpha Innotech Co., USA) after electrophoresis on 1% agarose gel (IBI Scientific, Peosta Lowa) containing 0.2 mg/ ml ethidium bromide at 80 volts using 1× TBE electrophoresis buffer (Bio Basic Inc. USA). For confirmation of identity of PCR amplicons gel purified (Mini Elute Gel Extraction kit, Qiagen, Germany) amplicons were custom sequenced (Eurofins Genomics Laboratory, India). DNA extracted from reference strain of B. bronchiseptica (MTCC 6838) available in the laboratory was used as positive control.
The study concludes that serum samples may be better sample for diagnosis of bordetellosis for identification of pathogens DNA instead of deep nasal swabs in dogs.
ACKNOWLEDGEMENT
Authors are thankful to Director IVRI, Izatnagar, Joint Director (Academics), and Joint Director (R) IVRI, Izatnagar for providing fellowship to Lenin, Monika and Prasannavadhna, and funds for undertaking the study. We are also thankful to the dog owners, veterinary institutions, and clinicians. Technical assistance by HC Joshi and Laik of Epidemiology Laboratory was instrumental in carrying out this study.
CONFLICT OF INTEREST
There is no conflict of interest among all or any of the authors and also with the funding agencies.
REFERENCES




