Advances in Animal and Veterinary Sciences
Review Article
Advances in Animal and Veterinary Sciences 2 (6): 329 – 336Kyasanur Forest Disease: a Status Update
Jeny Kalluvila John1*, Jobin Jose Kattoor2, Anoopraj Rajappan Nair1, Aswathi Plantharayi Bharathan3, Rekha Valsala4, Gurupriya Vijayasaraswathy Sadanandan5
- Department of Veterinary Pathology, Indian Veterinary Research Institute, Izatnagar, Bareilly, U.P
- Department of Virology, Indian Veterinary Research Institute, Izatnagar, Bareilly, U.P
- Central Avian Research Institute, Izatnagar, Bareilly, UP
- Department of Bacteriology and Mycology, Indian Veterinary Research Institute, Izatnagar, Bareilly, U.P
- Department of Animal Biochemistry, Indian Veterinary Research Institute, Izatnagar, Bareilly, U.P
*Corresponding author: jenykj@gmail.com
ARTICLE CITATION:
John JK, Kattoor JJ, Nair AR, Bharathan AP, Valsala R, Sadanandan GV (2014). Kyasanur forest disease: a status update. Adv. Anim. Vet. Sci. 2 (6): 329 – 336.
Received: 2014–05–26, Revised: 2014–07–04, Accepted: 2014–07–05
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.6.329.336
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Kyasanur Forest Disease (KFD) is an emerging zoonotic viral tick borne disease affecting mainly monkeys. The etiological agent of KFD is Kyasanur Forest Disease virus (KFDV), a RNA virus of the genus Flavivirus, family Flaviviridae. The natural host of KFDV mainly involves wild primates and various tick species of genus Haemaphysalis. In enzootic areas, the KFDV was maintained and circulated in small mammals especially rodents, shrews, ground birds and ticks. KFD is endemic in 5 areas of Karnataka, India mainly Shimoga, Chikkamagalore, Uttara Kannada, Dakshina Kannada, and Udupi. KFD has been reported also from Tamil Nadu and Kerala. A variant of KFD has been isolated from Saudi Arabia and China. The various isolates of KFDV from India, Saudi Arabia, and China share a recent common ancestor. Though the prevalence of KFD is reported from few areas, chances of existence of virus outside these foci can’t be eliminated. KFD should be included in differential diagnosis of diseases in other areas of Asia and Middle East The clinical manifestation of disease ranges from haemorrhagic phase to neurological manifestations. Diagnosis of KFD is mainly by virus isolation or by various serological detection methods. Molecular diagnostic methods such as RT–PCR, real time PCR are also available. A formalin inactivated tissue culture vaccine is available for prophylaxis. Other control strategy includes wearing protective clothing while handling infectious materials and tick control. Thorough knowledge of transmission of disease is very essential in control/prevention programmes. There is a requirement of better diagnostic methods, tick control strategies, public awareness, more area coverage of immunization and regular revaccination.
INTRODUCTION
Kyasanur Forest Disease (KFD) is an emerging zoonotic viral tick borne disease affecting mainly monkeys. Every year lots of human cases are reporting with a morbidity rate of around 2–10% in South India (Gould and Solomon, 2008). It was first noticed when cases of monkey mortality occurred in a forest area of Shimoga district, India, followed by acute, febrile haemorrhagic disease in humans nearby during 1957 (Work et al., 1959). Around 400–500 cases of KFD are reporting from India every year (Work et al., 1957; Pavri, 1989). As a tick borne infection, it has a seasonal occurrence from January to June. Monkeys and humans are the only known host species that build up clinical disease with KFD virus. KFD virus circulates through small mammals such as porcupines, squirrels, rodents, shrews and ground birds and also in tick species in the endemic areas (Pattnaik, 2006). Kyasanur forest disease is endemic in Karnataka, Tamil Nadu and Kerala (CDC, 2013). As prophylactic measure formalin inactivated tissue culture vaccine is used in the diseased areas. In spite of vaccination, every year new cases are reporting from these areas. The possible factor for emergence of new cases can be due to low coverage of the vaccine (Kasabi et al., 2013) or due to lack of proper control of tick in endemic areas. The present review attempts to summarize on the various aspects of disease, its etiology, transmission, clinical features, epidemiology, diagnosis and various control strategies.
ETIOLOGY
The etiological agent of KFD is Kyasanur Forest Disease virus (KFDV), a RNA virus of the genus Flavivirus, family Flaviviridae (Lin et al., 2003; Thiel et al., 2005). The virus is positive sense with single stranded RNA virus. The KFDV is spherical (40–65nm in size), enveloped virus with an icosahedral nucleocapsid. The genome of flavivirus has 3 structural proteins (Capsid, prM, and Envelope) and 8 non–structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5 and NS5B).The genomic RNA is similar to cellular mRNA except for not having poly–adenylated tail. The replication of Flaviviruses takes place in the cytoplasm of infected cells utilizing host cell’s polymerase (Kofler et al., 2006; Villordo, and Gamarnik, 2009).
Based on mode of transmission, the flaviviruses can be grouped into two: the vector borne viruses and the other with no known vector (Kuno et al., 1998). The vector borne viruses can be subdivided into a mosquito borne viruses and as tick borne viruses (Gaunt et al., 2001)
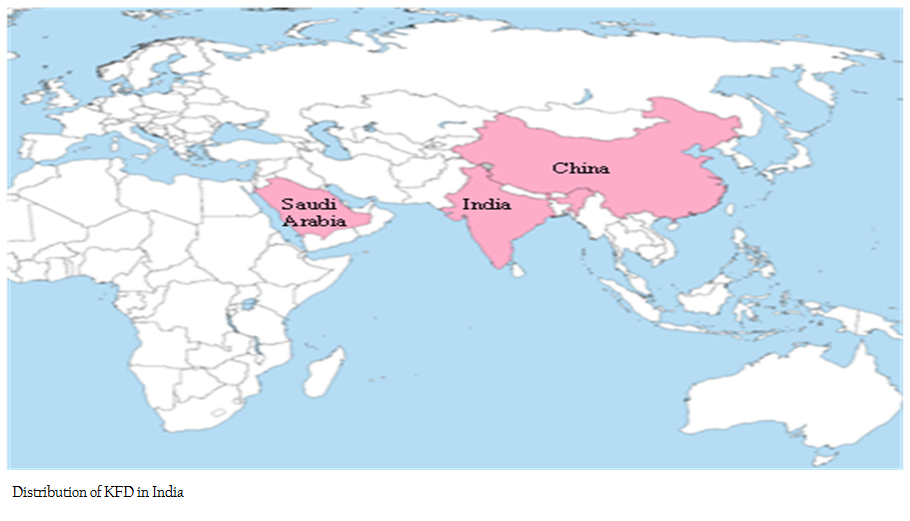
KFDV variants have been isolated from Saudi Arabia (Carletti et al., 2010; Memish et al., 2011) and China (Zhang et al., 1989; Zaki, 1997; Wang et al., 2009). During 1995 and 2001, a novel Flavivirus was isolated from haemorrhagic fever patients in Jeddah and Makkah area of Saudi Arabia (Qattan et al., 1996; Zaki, 1997; Madani, 2005; Madani et al., 2011). This novel flavivirus was initially isolated from Alkhumra district, Saudi Arabia, hence named as Alkhumra virus infection. The nucleotide analysis of prototype strain of this virus from Saudi Arabia (strain 1176) and the KFDV reference strain from India (P–9605) showed 92% sequence similarity. Charrel et al, (2007) isolated Alkhumra virus from Ornithodoros savignyi.
Another variant of KFDV was isolated from a febrile patient in south western China initially referred as Nanjianyin virus. Later studies grouped the Nanjianyin virus in KFDV group. The sequence alignment and homology analysis conducted by Wang et al., (2009) revealed that Nanjianyin virus belongs to the KFD virus clade and the results of phylogenetic analysis of PrM–E gene and NS5 gene suggesting that both viruses are in same genetic cluster.
Studies suggesting that tick born flaviviruses spread through various geographical areas are comparatively very slow (Gould et al., 2001; Gould and Solomon, 2008). The diversity and evolution study of KFDV by Mehla et al, (2009) point out that the various isolates of KFDV from India, Saudi Arabia, and China share a recent common ancestor.
HOST RANGE AND VECTOR INVOLVED
KFDV infection was reported mainly from wild primates and humans. The natural host of KFDV mainly involves wild primates: black faced langurs (Semnopithecus entellus) and red faced bonnet monkeys (Macaca radiata) and various tick species of genus Haemaphysalis (Work and Trapido, 1957; Bhatt et al., 1966). Many wild animals serve as natural hosts, the Blanford rat (Rattus blanfordi), the striped forest squirrel (Funambulus tristriatus tristriatus) and the house shrew (Suncus murinus). These animals have sufficient viremic titers for the transmission (Trapido et al., 1959).
Wide host range of KFDV includes humans, tick species, rodents (shrews, forest rats, white tailed rat, and white bellied rat), monkeys (grey langur, black–faced langur, and bonnet macaque), bats, ground dwelling birds, squirrels, Indian crested porcupines. In experimental infections with KFDV, high virus titers was noticed in black–napped hares, porcupines, flying squirrels, Malabar giant squirrels, three–striped squirrels, gerbils (Boshell, 1969). Domestic ruminants can maintain the infected tick population for long time.
The ticks of genus Haemophysalis, mainly Haemophysalis spinigera act as a major vector for KFD (Sreenivasan et al., 1986). Wide spread distribution of this species of tick in forests especially tropical and deciduous of southern and central India. KFDV has been isolated from various other species of ticks, H. turturis, H pauana kinneari, H. kyasanurensis, H. minuta, Dermacentor, Ixodes, Ornithodorus (adult), Hyalomma marginatum isaaci (Verma et al., 1960; Singh et al., 1964; Singh and Bhatt., 1968; Singh et al., 1968; Bhat and Naik, 1978; Bhat et al., 1978a).
In the transmission of KFDV, human act as a dead end host, with no sufficient viremia for further transmission (Labuda et al., 1993). Neutralizing antibodies of KFDV have been found in cattle, buffaloes, goats, wild boars, porcupines, squirrels, flying squirrels, rats, mice, shrews, bats (Bhatt et al., 1978b) and a number of bird species. Amplification of virus occurs in monkeys (Boshell, 1969).
MODE OF TRANSMISSION
The mode of evolution of KFDV is unclear, although in a study by Boshell (1969) reported, a considerable increase in the human population in Shimoga district during 1950’s. Increasing human needs for wood and land for agriculture leads to destruction of local forest areas (Boshell, 1969). Alteration of ecosystem occurred as a result of human intrusion may led the way for introduction of KFDV from its wild reservoir host to humans.
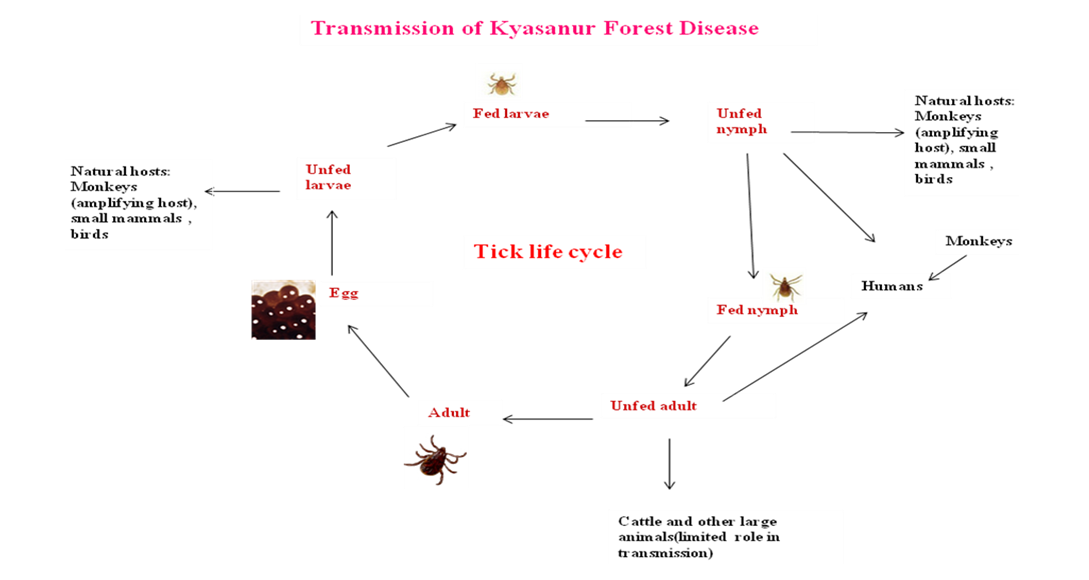
All tick–borne Flaviviruses share one general feature in its natural transmission cycle. Man having no role in virus transmission in any of these diseases. Humans do not develop adequate viremia to infect the ticks (Labuda et al., 1993). In KFD small mammals, mainly rodents have been considered as the reservoir. For the survival of many viruses, a reservoir is very essential. Rodents are best maintenance hosts; because of their short generation time, always provide a group of new animals. In ticks, virus is maintained throughout life, the virus is passed to next generation through transstadial and trans–ovarial transmission. Co–feeding of mammalian host offer a more convenient means for virus transmission among ticks than feeding a viremic animal (Boshell and Rajagopalan, 1968; Randolph, 2011)
In enzootic areas, the KFDV was maintained and circulated in small mammals especially rodents, shrews, ground birds and ticks (Pattnaik, 2006). Infection of wild monkeys occurs through the bite of infected ticks and further spread to other non infected ticks and monkeys. Severe febrile illness was noticed in some of the KFD infected monkeys. Human’s contract infection mainly through the bite of infected nymph and also by contact with infected animals especially monkeys. Horizontal transmission between humans not reported. Persons visiting forest for recreation or for collecting wood will contract infection by accidental tick bite (CDC, 2013) (Figure 1).
In primate host, black–faced langurs were found highly vulnerable to the virus (Sreenivasan et al., 1986). Tick population hike during dry season (December to May), results in all major epizootics during this period (Rajagopalan et al., 1968a, b). Viremic birds play an important role in distant spread of virus and may also carry tick infected with virus (Gould and Solomon, 2008).
RISK OF EXPOSURE
The KFD was found endemic in various districts of Karnataka, Tamil Nadu and Kerala. Additionally related KFDV was isolated from Saudi Arabia and China. Persons will get bite from infective ticks while visiting forest areas in Karnataka for recreation, hunting or for collecting wood and herbs. The disease has a seasonal occurrence mainly during dry periods (November– June). Visiting the forest areas of Karnataka during this period without adequate protective measures increases the risk of exposure. The environmental conditions favours the tick multiplication, makes an area endemic for KFD (Parola and Raoult, 2001). In recent years, increased occurrence of tick–transmitted diseases has been reported around the globe (Piesman and Eisen, 2008; Nicholson et. al., 2010). Grazing of cattle in forest areas with infected ticks led to a introduction of these ticks to new areas (Chomel et al. 2007).
EPIDEMIOLOGY
In the forest area of Shimoga district Karnataka, India during 1956 large number of monkey mortality were reported followed by acute, febrile haemorrhagic disease in humans nearby (Work et al., 1959). Research on this leads to the isolation of a new Flavivirus from the autopsied samples from monkeys. Later, an analogous virus was isolated from Ixodid ticks population in the affected forest areas (Work and Trapido, 1957). The name Kyasanur Forest disease was given after the forest where the first viral isolate was obtained (Kyasanur forest) (Dobler, 2010). Transmission is mainly by the tick of genus Haemaphysalis. Natural host of the virus are small wild mammals, become viremic and are infested by various stages of ticks (Trapido et al., 1959). Around 400–500 cases of KFD are reporting from India every year (Work et al., 1957).
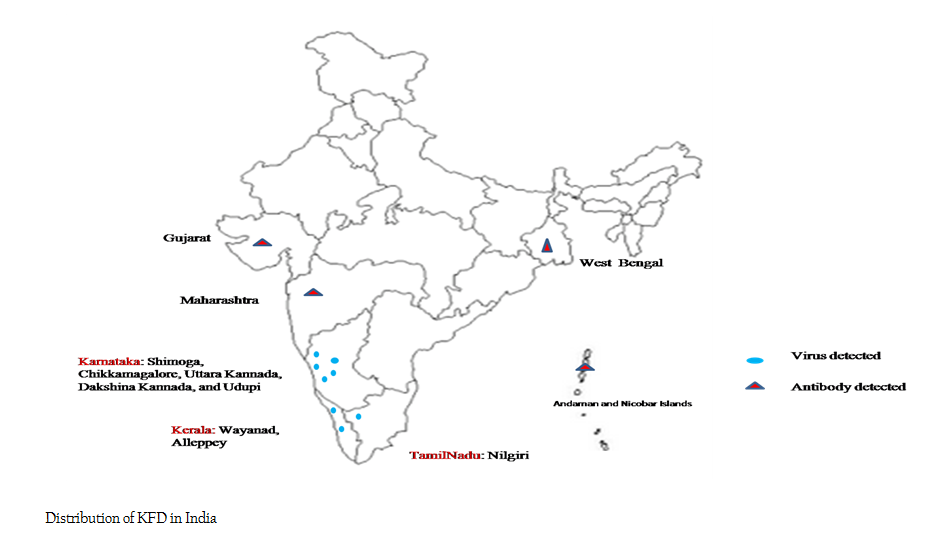
KFD is endemic in 5 areas of Karnataka, India mainly Shimoga, Chikkamagalore, Uttara Kannada, Dakshina Kannada, and Udupi. In every season of epidemic, around 500 cases are reporting from these areas (Sreenivasan et al., 1986; Pattnaik, 2006). Outbreaks in human population of Shimoga district, Mysore State were reported from 1959–1966 (Upadhyaya et al., 1975). Similarly reports of non human primates are from 1957 – 1964 by Goverdhan et al, (1974) and 1964–1973 by Sreenivasan et al, (1986). Report of Upadhyaya et al. (1975), mainly centered on endemic areas, whereas the report of Sreenivasan (1986) spread out the area to non endemic places also and reported 1046 monkey mortality during the peak season of tick activity. More cases around 50% are reported from black faced langurs.
During 2003, a total of 953 suspected cases were reported from human patients, out of which 306 were confirmed and 132 suspected cases from non human primates and out of that 11 were confirmed as KFD cases from Karnataka. During 2004, out of 568 suspected cases, 153 were confirmed in humans and out of 86 cases reported, 8 were confirmed as KFD in non human primates. From 2005–2008, a total of 1208 cases were reported from Shimoga district out of which 212 were positive for KFDV in humans. Between 2009 and 2011, a total of 225 suspected cases from humans, 83 were confirmed. The case fatality rate from 2003– 2012 is around 3.4%. During this period, maximum cases reported during 2003 and the least in 2007 and 2010. Case fatality rate of KFD in non human primates from 2003–2012 in Shimoga district is 1.4% (Holbrook, 2012).
In a study by Kasabi et al. (2013) reported 215 suspected cases from different villages of Shimoga from December 2011–March 2012, in that 61 were KFD positive. More cases are reported from adult males of those areas. In 2012 from Bandipur National Park, Karnataka State, 12 out of 21 suspected cases in humans, 4 monkeys (total death 12) and 2 out of 14 tick pools were confirmed as KFDV cases (Mourya et al., 2012). This study confirmed the spread of KFDV to new foci. Detection of KFDV in Tamil Nadu and Kerala State of India, pointing out the presence of the virus in several tropical forest areas of India. Serological evidences are there for the probable existence of KFDV in different states of India (Sarkar and Chatterjee, 1962; Pattnaik, 2006).
KFDV variants have been isolated from Saudi Arabia and China (Zaki, 1997; Wang et al., 2009). During 1995 and 2001, a novel flavi virus was isolated from haemorrhagic fever patients in Jeddah and Makkah area of Saudi Arabia (Zaki, 1997; Madani, 2005; Alzahrani et al., 2010). This novel flavivirus was initially isolated from Alkhumra district, Saudi Arabia, hence named as Alkhumra virus infection. Another variant of KFDV was isolated from a febrile patient in south western China initially referred as Nanjianyin virus. Later studies grouped the Nanjianyin virus in KFDV group.
Incidences of KFD in monkey were also confirmed in Nilgiris district of Tamilnadu. One incidence of Human was confirmed in Kerala State from Noolpuzha–Aalathoor colony in Wayanad district in 2013. Later in April 2014, the dreaded KFD has been diagnosed among monkeys of the temple compound at Vallikkavu near Chengannur in Kerala's Alappuzha district. Distribution of KFD in India and world are depicted (Figure 2a and Figure 2b)
CLINICAL SIGNS AND PATHOLOGY IN HUMANS
The incubation period of KFDV ranges from a few days up to 1week (Work and Trapido, 1957). KFD is endemic in 5 areas of Karnataka, India mainly Shimoga, Chikkamagalore, Uttara Kannada, Dakshina Kannada, and Udupi. (Pavri, 1989). The disease has various stages in development. The initial prodomal stage lasts for around one week, with sudden onset of fever, chills, headache, gastro intestinal disturbances, insomnia, sore throat, decreased blood pressure and heart rate, pain in muscles, extremities and conjunctivitis. Humans infected with KFDV have low platelet, white blood cells and red blood cells count. Ophthalmic manifestations of KFD are haemorrhages in conjunctiva, retina and vitreous humour, keratitis, opacity of lens, mild iritis (Iyer et al., 1959; Grard et al., 2007) The next haemorrhagic stage is characterised by irregular epistaxis with blood in vomitus and faeces, blisters on mouth, haemorrhages from the gum and nose. The haemorragic stage is followed by a long convalescent stage. Frequently, a second febrile stage (relapse phase– 10–20%) of around 2 weeks with same clinical manifestations of first phase along with various neurological complications was reported. Abnormal reflexes, confusion and tremors noticed as neurological complication (Pavri, 1989; Adhikari Prabha et al., 1993; Heymann, 2004; Pattnaik, 2006). Gross and histopathological lesions in KFD are not pathognomonic. Nephrosis, hepatomegaly with degenerative changes, pneumonia with haemorrhage in the lung parenchyma, haemorrhages in gastro intestinal tract, distinct reticulo–endothelial cells in liver and spleen, with noticeable phagocytosis of RBC in spleen (Pattnaik, P. (2006). No clear brain lesions were found on autopsy examination except cerebral oedema in few cases (Work et al., 1957).
CLINICAL SIGN
KFD in animals is always fatal with an acute onset. Mortality in animals is noticed during the high viremic stage. Case fatality rate of around 100% noticed in experimental infections (Kenyon et al., 1992). Clinical signs and pathology of KFD in bonnet macaque are similar to that of humans (Webb and Chaterjea, 1962). Neurological signs were noticed in second febrile stage in bonnet macaques in experimental infection with KFDV (Webb and Burston, 1966). Even though Rhesus macaque has a related viremic phase like that of bonnet macaque, there is no clinical illness/mortality noticed in this species (Work, 1958).
DIAGNOSIS
The clinical signs of KFD are similar to many other viral/ haemorrhagic fevers. There should be a reliable and fast differential diagnostic test for confirmation of KFD. The disease should be differentially diagnosed from various types of influenza’s, typhoid and from various rickettsial fevers (Mourya et al., 2014). Earlier for KFD detection, virus isolation and some antibody based detection methods such as hemagglutination inhibition (HI), complement fixation (CF) and neutralization test (NT) were used (Upadhyaya and Murthy, 1967; Pavri and Anderson, 1970). With advancement of technologies, laboratories developed various molecular diagnostic methods for diagnosis. Due to aerosol transmission of KFDV many cases were reported among the laboratory technicians. A BSL–3 facility is required for handling and working with KFDV, so during initial periods of outbreaks, little studies were conducted on KFD (Mourya et al., 2014). In India, many flavivirus infections are prevalent; cross reactivity between these viruses may create problems in diagnosis. Blood and serum samples should be collected aseptically from patients with necessary protective measures for the personnel’s collecting samples. Complete blood picture analysis should be carried out in blood samples of suspected patients. Also various liver and kidney function tests (Mourya et al., 2014). The virus isolation can be done from blood during febrile period or from organ samples collected during autopsy. Paired sera sample can be used for serological examination.
Virus Isolation
Virus isolation of KFDV can be done in BHK–21, Vero E6 cell lines, embryonated chick cell or in mice (Mehla et al., 2009; Wang et al., 2009). In BHK–21, KFDV will produce characteristic cytopathic effect. Intra–cerebral inoculation of virus in 3 day old mice will cause mortality in all. Similar findings were obtained after intra–peritoneal inoculation in 50 day old mice (Wang et al., 2009). Mice (3 day old) are highly recommended for virus isolation than all other methods due to its high vulnerability to virus (Mourya et al., 2014).
Serological Methods
By HI test and neutralization test, KFDV antibodies were demonstrated from many states of India especially from south western states such as Gujarat and Maharashtra, also from West Bengal and Andaman and Nicobar Islands. In Andaman and Nicobar Islands, prevalence of KFD is around 12% when detected by neutralization test. (Padbidri et al., 2002). Mourya et al., (2012) developed IgM capture ELISA for detection of infection during acute phase.
Molecular Diagnosis
RT–PCR and real time PCR provides a very rapid and accurate diagnosis (Mackay et al., 2002; Mehla et al., 2009; Mourya et al., 2012). The RT–PCR reactions are highly specific and sensitive compared to other conventional methods (Eldadah et al., 1991; Tanaka, 1993; Fulmali, 2012). Mourya et al., (2012) developed nested RT–PCR, real–time RT–PCR for the rapid detection of KFD during acute phase infection. The flaviviruses specific NS–5 region was targeted for primer designing.
LABORATORY HAZARDS
Inhalation of aerosols may be the most frequent way of acquiring infection between persons handling the infected materials. Other means of transmission includes while conducting post mortem examination, accidental parentral inoculation, spilling out of contents from broken glasswares or accidental ingestion (Morse et al., 1962; Banerjee et al., 1979). One should follow the WHO guideline while shipping of samples for diagnosis (WHO, 2013).
TREATMENT
Currently, no specific antiviral treatment exists for KFDV in humans; early hospitalization and supportive treatment becomes more essential. Supportive therapy includes the maintenance of normal blood cell counts, blood pressure and hydration (CDC, 2013; Mourya et al., 2014). Also, pain reliefs, antipyretics, blood transfusion, and antimicrobial therapy for secondary infections, corticosteroids and anticonvulsants for nervous disorders (Adhikari Prabha et al., 1993; Boria et al., 2002).
PREVENTION AND CONTROL
Tick borne diseases are emerging as a result of changes in public health policy, acaricide resistance, climatic changes, and emergence of new variant of pathogen. Measures need to be taken for reversal of these conditions (Dhama et al., 2013a). Prevention strategies such as quarantine, vaccination, early diagnosis, tick control will restrict the entry of virus to new areas. The KFDV has been classified as risk group IV pathogen. Vaccination is one of the main control strategies for KFD. Formalin inactivated tissue culture vaccines are available for immunization against KFDV in endemic areas. Other control strategy includes wearing protective clothing while handling infectious materials and tick control. Strictly prohibit the visit to affected forest areas during outbreak time. If visit is inevitable, use protective clothing’s and gum boots to cover the whole body and apply some insect repellent to exposed body part (Ghosh et al., 2006; Piesman and Eisen, 2008; Kilpatrick et al., 2012; Mourya et al., 2014). The concept of ‘one world, one health and one medicine’ should be kept in mind while combating zoonotic infections (Dhama et al., 2013b).
VACCINES
An inactivated/killed tissue culture vaccine has been used in endemic areas of Karnataka, India since 1990. Initially 2 doses were used in persons of 7– 65 years of age, in an interval of 4 weeks. Revaccination is required after 6–9 months for five years (Kasabi et al., 2013). Before introduction of formalin inactivated tissue culture vaccine, several other live and inactivated vaccines prepared in tissue culture, formalin–inactivated Russian Spring Summer Encephalitis (RSSE) virus (Aniker et al., 1962; Shah et al., 1962) and mouse brain were used in control programs (Bhatt and Anderson, 1971; Upadhyaya et al., 1979). Incidence of KFD has been reported even in vaccinated individuals. The main drawback of these vaccines is poor area coverage, not taking boosters and poor storage conditions. Utilizing new technologies of vaccine production, develop better vaccine will combat the infection (Dhama et al., 2008; Paul–Pierre, 2009; Dhama et al., 2013c) Vaccines against KFDV were initially produced in Shimoga district of Karnataka. Later, the unit was moved to Bangalore (Institute of Animal Husbandry and Veterinary Biologicals, Hebbal). In a study conducted by Kasabi et al, (2013) noticed low coverage of vaccine in affected areas even less than half of the target population and the efficiency of vaccine was around 62% in individuals received initial 2 doses and 83% in individuals who received further boosters. In earlier studies, reported the efficiency around 79% in persons with one dose and 94% in those received 2 doses (Dandawate et al., 1994) and about 59% in those have 2 doses during 1970–1971 (Upadhyaya et al., 1979, Dandawate et al., 1980).
CONCLUSION
Kyasanur Forest Disease (KFD) is an emerging zoonotic viral tick borne disease affecting mainly monkeys. Every year from South India hundreds of human cases are reporting. Being a tick borne disease, strict measures should be taken for controlling the tick population. Formalin inactivated tissue culture vaccine are available for immunization in affected areas. Regular vaccination should be carried out for consecutive five years with increased area coverage. Even though an effective vaccine is available, KFD is still widespread and remain as a source of infection for humans. There is urgent need of effective control strategies so that this type of tick borne infections can be controlled.
REFERENCES
Adhikari Prabha MR, Prabhu MG, Raghuveer CV, Bai M, Mala MA (1993). Clinical study of 100 cases of Kyasanur Forest disease with clinicopathological correlation. Indian J. Med. Res. 47 (5): 124 – 130.
Alzahrani AG, Al Shaiban HM, Al Mazroa MA, Al–Hayani O, Macneil A, Rollin PE, Memish ZA (2010). Alkhurma hemorrhagic fever in humans, Najran, Saudi Arabia. Emerg. Infect. Dis. 16:1882 – 1888
http://dx.doi.org/10.3201/eid1612.100417
PMid:21122217 PMCid:PMC3294564
Aniker SP, Work TH, Chandrasekharaiya T (1962). The administration of formalin–inactivated RSSE virus vaccine in the kyasanur forest disease area of Shimoga district, Mysore state. Indian J. Med. Res. 50: 147 – 152.
PMid:13861642
Banerjee K, Gupta NP, Goverdhan MK (1979). Viral infections in laboratory personnel. Indian J. Med. Res. 69 (3): 363 – 373.
PMid:447375
Bhat HR, Naik S V, Ilkal MA, Banerjee K (1978a). Transmission of Kyasanur forest disease virus by Rhipicephalus haemaphysaloides ticks. Acta. Virol. 22 (3): 241 – 244.
PMid:27975
Bhat HR, Naik SV (1978). Transmission of Kyasanur forest disease virus by Haemaphysalis wellingtoni Nuttall and Warburton, 1907 (Acarina :Ixodidae). Indian J. Med. Res. 67:697 – 703.
PMid:680913
Bhat HR, Sreenivasan MA, Goverdhan MK, Naik SV, Banerjee K (1978b). Antibodies to Kyasanur forest disease virus in bats in the epizootic–epidemic area and neighbourhood. Ind. J. Med. Res. 68: 387 – 392.
PMid:217820
Bhatt PN, Anderson CR (1971). Attenuation of a strain of Kyasanur Forest disease virus for mice. Indian J. Med. Res. 59: 199–205.
PMid:5579236
Bhatt PN, Work TH, Varma MGR, Trapido H, Narasimha MDP, Rodrigues FM (1966). Isolation of Kyasanur forest disease from infected humans and monkeys of Shimoga District, Mysore State. Indian J. Med. Res. 20: 316–320.
Boshell J (1969). Kyasanur Forest disease: ecologic considerations. Am. J. Trop. Med. Hyg. 18: 67–80.
PMid:5812658
Boshell JM, Rajagopalan PK (1968). Observations on the experimental exposure to the monkeys, rodents and shrews to infestation of ticks in forest of Kyasanur forest disease area. Indian J. Med. Res. 56: 586–588.
Carletti F, Castilletti C, Di Caro A, Capobianchi MR, Nisii C, Suter F, Rizzi M, Tebaldi A, Goglio A, Passerini Tosi C, Ippolito G (2010). Alkhurma hemorrhagic fever in travelers returning from Egypt. Emerg. Infect. Dis. 16: 1979–1982.
http://dx.doi.org/10.3201/eid1612.101092
PMid:21122237 PMCid:PMC3294557
CDC (2013). Kyasanur Forest disease. Available at: http://www.cdc.gov/vhf/kyasanur. Accessed on June 2014.
Charrel RN, Fagbo S, Moureau G, Alqahtani MH, Temmam S, de Lamballerie X (2007). Alkhurma hemorrhagic fever virus in Ornithodoros savignyi ticks. Emerg. Infect. Dis. 13:153 – 155.
http://dx.doi.org/10.3201/eid1301.061094
PMid:17370534 PMCid:PMC2725816
Chomel BB, Belotto A, Meslin FX (2007). Wildlife, Exotic Pets, and Emerging Zoonoses. Emerg. Infect. Dis. 13(1): 6 – 11.
http://dx.doi.org/10.3201/eid1301.060480
PMid:17370509 PMCid:PMC2725831
Dandawate CN, Desai GB, Achar TR, Banerjee K (1994). Field evaluation of formalin inactivated Kyasanur forest disease virus tissue culture vaccine in three districts of Karnataka state. Indian J. Med. Res. 99: 152–158.
PMid:7927566
Dandawate CN, Upadhyaya S, Banerjee K (1980). Serological response to formalized Kyasanur Forest disease virus vaccine in humans at Sagar and Sorab Talukas of Shimoga district. J. Biol. Stand. 8:1–6.
http://dx.doi.org/10.1016/S0092-1157(80)80041-2
Dhama K, Mahendran M, Gupta PK, Rai A (2008). DNA vaccines and their applications in veterinary practice: Current perspectives. Vet. Res. Commun. 32:341 – 356.
http://dx.doi.org/10.1007/s11259-008-9040-3
PMid:18425596
Dhama K, Tiwari R, Chakraborty S, Kumar A, Karikalan M, Singh R Rai RB (2013a). Global warming and emerging infectious diseases of animals and humans: current scenario, challenges, solutions and future perspectives – a review. Int. J. Curr. Res. 5(07): 1942–1958.
Dhama K, Chakraborty S, Kapoor S, Tiwari R, Kumar A, Deb R, Rajagunalan S, Singh R, Vora K, Natesan S (2013b).One world, one health– veterinary perspectives. Adv. Anim. Vet. Sci. 1: 5 – 13.
Dhama K, Chakraborty S, Wani MY, Tiwari R, Barathidasan (2013c). Cytokine therapy for compating animal and human diseases: A review. Res. Opin. Anim. Vet. Sci. 3: 195 – 208.
Dobler G (2010). Zoonotic tick–borne flaviviruses. Vet Microbiol. 140: 221–228.
http://dx.doi.org/10.1016/j.vetmic.2009.08.024
PMid:19765917
Eldadah ZA, Asher DM, Godec MS, Pomeroy KL, Goldfarb LG, Feinstone SM, Levitan H, Gibbs CJ Jr, Gajdusek DC (1991). Detection of flaviviruses by reverse–transcriptase polymerase chain reaction. J. Med. Virol. 33: 260 – 267.
http://dx.doi.org/10.1002/jmv.1890330410
PMid:1713265
Fulmali PV (2012). Priorities and future of diagnosis of emerging viral diseases. Health Sciences. 1(1).
Gaunt MW, Sall AA, de Lamballerie X, Falconar AK, Dzhivanian TI, Gould EA (2001). "Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography". J. Gen. Virol. 82 (8): 1867–1876.
PMid:11457992
Ghosh S, Azhahianambi P, Fuente J (2006). Control of ticks of ruminants, with special emphasis on livestock farming systems in India: present and future possibilities for integrated control—a review. Experiment. Appl. Acarol. 40: 49 – 66.
http://dx.doi.org/10.1007/s10493-006-9022-5
PMid:17004030
Gould EA, Solomon T (2008). Pathogenic flaviviruses. Lancet. 371: 500–509.
http://dx.doi.org/10.1016/S0140-6736(08)60238-X
Gould EA, de Lamballerie X, Zanotto PM, Holmes EC (2001). Evolution, epidemiology, and dispersal of flaviviruses revealed by molecular phylogenies. Adv. Virus. Res. 57:71–103.
http://dx.doi.org/10.1016/S0065-3527(01)57001-3
Goverdhan MK, Rajagopalan PK, Narasimha Murthy DP, Upadhyaya S, Boshell MJ, Trapido H, Ramachandra Rao T (1974). Epizootiology of Kyasanur Forest Disease in wild monkeys of Shimoga district, Mysore State (1957–1964). Indian J. Med. Res. 62: 497–510.
PMid:4215749
Grard G, Moureau G, Charrel RN, Lemasson JJ, Gonzalez JP, Gallian P, Gritsun TS, Holmes EC, Gould EA, de Lamballerie X (2007). Genetic characterization of tick–borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology. 361 (1): 80–92.
http://dx.doi.org/10.1016/j.virol.2006.09.015
PMid:17169393
Heymann DL (2004). An Official Report of the American Public Health Association. In D. L. Heymann (Ed.), Control of Communicable Diseases Manual. (18th ed) Washington, D.C. American Public Health Association. 35 – 37.
Holbrook MR (2012). Kyasanur Forest Disease. Antiviral Res. 96(3): 353 – 362
http://dx.doi.org/10.1016/j.antiviral.2012.10.005
PMid:23110991 PMCid:PMC3513490
Iyer CG, Laxmana Rao R, Work TH, Narasimha Murthy DP (1959). Kyasanur Forest Disease VI. Pathological findings in three fatal human cases of Kyasanur Forest Disease. Indian J. Med. Sci. 13:1011 – 1022
PMid:14406181
Kasabi GS, Murhekar MV, Sandhya VK, Raghunandan R, Kiran SK, Sandhya VK, Channabasappa GH, Mishra AC, Mourya DT, Mehendale SM (2013). Coverage and Effectiveness of Kyasanur Forest Disease (KFD) Vaccine in Karnataka, South India, 2005 – 10. PLoS Negl. Trop. Dis. 7(1): e2025.
http://dx.doi.org/10.1371/journal.pntd.0002025
PMid:23359421 PMCid:PMC3554520
Kenyon RH, Rippy MK, McKee KT Jr, Zack PM, Peters CJ (1992). Infection of Macaca radiata with viruses of the tick–borne encephalitis group. Microb. Pathog. 13(5): 399 – 409.
http://dx.doi.org/10.1016/0882-4010(92)90083-Z
Kilpatrick AM, Randolph SE (2012). Drivers, dynamics, and control of emerging vector–borne zoonotic diseases. Lancet. 380(9857): 1946 – 1955.
http://dx.doi.org/10.1016/S0140-6736(12)61151-9
Kofler RM, Hoenninger VM, Thurner C, Mandl CW (2006). Functional analysis of the tick–borne encephalitis virus cyclization elements indicates major differences between mosquito–borne and tick–borne flaviviruses. J. Virol. 80 (8): 4099 – 4113.
http://dx.doi.org/10.1128/JVI.80.8.4099-4113.2006
PMid:16571826 PMCid:PMC1440478
Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB (1998). "Phylogeny of the genus Flavivirus". J. Virol. 72 (1): 73 – 83.
PMid:9420202 PMCid:PMC109351
Labuda M, Danielova V, Jones LD, Nuttal PA (1993). Amplification of tick–borne encephalitis virus infection during co–feeding of ticks. Med. Vet. Entomol. 7: 339 – 342.
http://dx.doi.org/10.1111/j.1365-2915.1993.tb00702.x
PMid:8268488
Lin D, Li L, Dick D, Shope RE, Feldmann H, Barrett AD, Holbrook MR (2003). Analysis of the complete genome of the tick–borne flavivirus Omsk hemorrhagic fever virus. Virology. 313: 81–90.
http://dx.doi.org/10.1016/S0042-6822(03)00246-0
Mackay IM, Arden KE, Nitsche A (2002). Real–time PCR in virology. Nuc Acids Res. 30:1292 – 305.
http://dx.doi.org/10.1093/nar/30.6.1292
PMid:11884626 PMCid:PMC101343
Madani TA (2005). Alkhumra virus infection, a new viral hemorrhagic fever in Saudi Arabia. J. Infect. 51(2): 91 – 97.
http://dx.doi.org/10.1016/j.jinf.2004.11.012
PMid:16038757
Madani TA, Azhar EI, Abuelzein el–TM, Kao M, Al–Bar HM, Abu–Araki H, Niedrig M, Ksiazek TG (2011). Alkhurma virus outbreak in Najran, Saudi Arabia: epidemiological, clinical, and Laboratory characteristics. J. Infection. 62: 67 – 76.
http://dx.doi.org/10.1016/j.jinf.2010.09.032
PMid:20920527
Mehla R, Kumar SRP, Yadav P, Barde PV, Yergolkar PN, Erickson BR, Carroll SA, Mishra AC, Nichol ST, Mourya DT (2009). Recent Ancestry of Kyasanur Forest Disease Virus. Emerg. Infect. Dis. 15 (9): 1431 – 1437.
http://dx.doi.org/10.3201/eid1509.080759
PMid:19788811 PMCid:PMC2819879
Memish ZA, Albarrak A, Almazroa MA, Al–Omar I, Alhakeem R, Assiri A, Fagbo S, MacNeil A, Rollin PE, Abdullah N, Stephens G (2011). Seroprevalence of Alkhurma and other hemorrhagic fever viruses, Saudi Arabia. Emerg. Infect. Dis. 17:2316–2318.
http://dx.doi.org/10.3201/eid1712.110658
PMid:22172587 PMCid:PMC3311215
Morse LJ, Russ SB, Needy CF, Buescher EL (1962). Studies of viruses of the tick–borne encephalitis complex. II. Disease and immune responses in man following accidental infection with Kyasanur Forest disease virus. J. Immunol. 88: 240 – 248.
PMid:14476341
Mourya DT, Yadav PD, Mehla R, Barde PV, Yergolkar PN, Kumar SRP, Thakare JP, Mishra AC (2012). Diagnosis of Kyasanur Forest disease by nested RT–PCR, real–time RT–PCR and IgM capture ELISA. J. Virol. Methods. 186:49 – 54.
http://dx.doi.org/10.1016/j.jviromet.2012.07.019
PMid:22874757
Mourya DT, Yadav PD, Patil DY (2014). Highly infectious tick borne viral diseases: Kyasanur forest disease and Crimean–Congo hemorrhagic fever in India. WHO South–East Asia J. Public Health. 3(1): 8 – 21.
Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE (2010). The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 26:205–212.
http://dx.doi.org/10.1016/j.pt.2010.01.007
PMid:20207197
Padbidri VS, Wairagkar NS, Joshi GD, Umarani UB, Risbud AR, Gaikwad DL, Bedekar SS, Divekar AD, Rodrigues FM (2002). A serological survey of arboviral diseases among the human population of the Andaman and Nicobar Islands, India. Southeast Asian J Trop Med Public Health. 33: 794–800.
PMid:12757228
Parola P, Raoult D (2001). Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32:897 – 928.
http://dx.doi.org/10.1086/319347
PMid:11247714
Pattnaik P (2006). Kyasanur forest disease: An epidemiological view in India. Rev. Med. Virol. 16 (3): 151 – 165.
http://dx.doi.org/10.1002/rmv.495
PMid:16710839
Paul–Pierre P (2009). Emerging diseases, zoonoses and vaccines to control them. Vaccine. 27: 6435 – 6438.
http://dx.doi.org/10.1016/j.vaccine.2009.06.021
PMid:19559117
Pavri K (1989). Clinical, clinicopathologic, and hematologic features of Kyasanur forest disease. Rev. Infect. Dis. 11: 854.
http://dx.doi.org/10.1093/clinids/11.Supplement_4.S854
Pavri KM, Anderson CR (1970). Serological response of man to Kyasanur Forest disease. Indian J. Med. Res. 58: 1587 – 607.
PMid:5505348
Piesman J, Eisen L (2008). Prevention of tick–borne diseases. Annu. Rev. Entomol. 53:323 – 343.
http://dx.doi.org/10.1146/annurev.ento.53.103106.093429
PMid:17877457
Qattan I, Akbar N, Afif H, Abu Azmah S, Al–Khateeb T, Zaki A (1996). A novel flavivirus: Makkah Region, 1994–1996. Saudi Epidemiology Bulletin. 3:1 – 3.
Rajagopalan PK, Patil AP, Boshell J (1968a). Ixodid ticks on their mammalian hosts in the Kyasanur Forest disease area of Mysore State, India, 1961–64. Indian J. Med. Res. 56:510 – 525.
PMid:4971268
Rajagopalan PK, Patil AP, Boshell J (1968b). Studies on Ixodid tick populations on the forest floor in the Kyasanur Forest disease area. Randolph SE (2011). Transmission of tick–borne pathogens between co–feeding ticks: Milan Labuda's enduring paradigm. Ticks. Tick Borne Dis. 2:179 – 182.
Sarkar JK. Chatterjee SN (1962). Survey of antibodies against arthropod–borne viruses in the human sera collected from Calcutta and other areas of West Bengal. Ind. J. Med. Res. 50: 833 – 841.
PMid:13991507
Shah KV, Aniker SP, Murthy DP, Rodrigues FM, Jayadeviah MS, Prasanna HA (1962). Evaluation of the field experience with formalin–inactivated mouse brain vaccine of Russian spring–summer encephalitis virus against Kyasanur Forest disease. Indian J. Med. Res. 50: 162 – 174.
PMid:13911122
Singh KR, Bhatt PN (1968). Transmission of Kyasanur Forest disease virus by Hyalomma marginatum isaaci. Ind. J. Med. Res. 56: 610 – 613.
PMid:5679153
Singh KR, Goverdhan MK, Rao TR (1968). Experimental transmission of Kyasanur forest disease virus to small mammals by Ixodes petauristae, I. ceylonensis and Haemaphysalis spinigera. Ind. J. Med. Res. 56:594 – 609.
PMid:5679152
Singh KR, Pavri K, Anderson CR (1964). Transmission of Kyasanur Forest disease by Haemaphysalis tururis, Haemaphysalis papuanakinneari and Haemaphysalis minuta. Ind. J. Med. Res. 52: 566 – 573.
PMid:14184087
Sreenivasan MA, Bhat HR, Rajagopalan PK (1986). The epizootics of Kyasanur Forest disease in wild monkeys during 1964 to 1973. Trans. R. Soc. Trop. Med. Hyg. 80(5): 810 – 814.
http://dx.doi.org/10.1016/0035-9203(86)90390-1
Tanaka M (1993). Rapid identification of flavivirus using the polymerase chain reaction. J. Virol. Methods. 41: 311–322.
http://dx.doi.org/10.1016/0166-0934(93)90020-R
Thiel H–J, Collett MS, Gould EA, Heinz FX, Houghton M, Meyers G (2005). Flaviviridae In: Fauquet CM, Mayo MA, Maniloff J, Desslberger U, Ball LA. Virus taxonomy: Eighth report of the International Committee on Taxonomy of Viruses. San Diego (CA): Elsevier Academic Press, 981 – 998.
Trapido H, Rajagopalan PK, Work TH, Varma MGR (1959). Kyasanur Forest disease. VIII. Isolation of Kyasanur Forest disease virus from naturally infected ticks of the genus Haemaphysalis. Indian J. Med. Res. 47: 133 – 138
PMid:13653739
Upadhyaya S, Narasimha Murthy DP, Anderson CR (1975). Kyasanur forest disease in the human population of Shimoga district, Mysore state (1959–1966). Indian J. Med. Res. 63:1556 – 63
PMid:1222964
Upadhyaya S, Dandawate CN, Banerjee K (1979). Surveillance of formolized KFD virus vaccine administration in Sagar–Sorab talukas of Shimoga district. Indian J. Med. Res. 69: 714 – 719.
PMid:511251
Upadhyaya S, Murthy DP (1967). Kyasanur Forest Disease virus neutralizing antibodies in human sera in Mysore State. Indian J. Med. Res. 55(2): 103 – 108.
PMid:6045044
Verma MG, Webb HE, Pavri K (1960). Studies on the transmission of Kyasanur Forest disease virus by Haemaphysalis spinigera Newman. Transfusion (Paris). 54:509 – 516.
Villordo SM, Gamarnik AV (2009). Genome cyclization as strategy for flavivirus RNA replication. Virus Res. 139 (2): 230 – 239.
http://dx.doi.org/10.1016/j.virusres.2008.07.016
PMid:18703097
Wang J, Zhang H, Fu S, Wang H, Ni D, Nasci R (2009). Isolation of Kyasanur forest disease virus from febrile patient, Yunnan, China. Emerg. Infect. Dis. 15:326 – 8.
http://dx.doi.org/10.3201/eid1502.080979
PMid:19193286 PMCid:PMC2657630
Webb HE, Burston J (1966). Clinical and pathological observations with special reference to the nervous system in Macaca radiata infected with Kyasanur Forest Disease virus. Trans. R. Soc. Trop. Med. Hyg. 60(3): 325 – 331.
http://dx.doi.org/10.1016/0035-9203(66)90296-3
Webb HE, Chaterjea JB (1962). Clinico–pathological observations on monkeys infected with Kyasanur Forest disease virus, with special reference to the haemopoietic system. Br. J. Haematol. 8: 401 – 413.
http://dx.doi.org/10.1111/j.1365-2141.1962.tb06544.x
PMid:13999331
Work TH (1958). Russian spring summer virus in India. Kyasanur forest disease. Prog. Med. Virol. 1: 248 – 279.
PMid:13579010
Work TH, Roderiguez FM, Bhatt PN (1959). Virological epidemiology of the 1958 epidemic of Kyasanur Forest disease. Am. J. Public Health Nations Health. 49: 869 – 74.
http://dx.doi.org/10.2105/AJPH.49.7.869
PMid:13661478 PMCid:PMC1372906
Work TH, Trapido H (1957). Kyasanur Forest disease, a new virus disease in India. Indian J. Med. Sci. 11:341.
PMid:13448774
Work TH, Trapido H, Narashima Murthy DP, Laxmana Rao R, Bhatt PN, Kulkarni KG (1957). Kyasanur Forest Disease III: A preliminary report on the nature of the infection and clinical manifestations in human beings. Indian J. Med. Sci. 11:619 – 645.
PMid:13474777
World health organization (WHO) (2013). A guide for shippers of infectious substances. Geneva. http://www.who.int/entity/ihr/e-Guide_2013.ppsx – accessed June 2014.
Zaki AM (1997). Isolation of a flavivirus related to the tick–borne encephalitis complex from human cases in Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 91:179 – 181.
http://dx.doi.org/10.1016/S0035-9203(97)90215-7
Zhang TS, Wang YM, Zhang YH, Duan S (1989). A survey of antibodies to arboviruses in residents of southwestern Yunnan Province (in Chinese). Chin. J. Endemiology. 10:74 – 77.