Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (5): 282 – 286Efficacy of Disinfectants in Inactivating H5N1 Avian Influenza Virus in Poultry Feces
Baleshwari Kurmi1, Harshad V Murugkar2, Manu Dixit3*, Shanmuga Nagarajan2, Diwaker D Kulkarni2, Manoj Kumar2
- Department of Veterinary Public Health & Epidemiology, College of Veterinary Science & A. H., Rewa, M.P., India
- High Security Animal Disease Laboratory, Bhopal, IVRI, Anand Nagar, Bhopal, M.P., India
- Office of Deputy Director Veterinary Services, Rewa, M.P., India.
*Corresponding author: manu.dixit@live.in
ARTICLE CITATION:
Kurmi B, Murugkar HV, Dixit M, Nagarajan S, Kulkarni DD, Kumar M (2014). Efficacy of disinfectants in inactivating H5N1 avian influenza virus in poultry feces. Adv. Anim. Vet. Sci. 2 (5): 282 – 286.
Received: 2014–03–31, Revised: 2014–05–10, Accepted: 2014–05–11
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.5.282.286
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Avian Influenza virus subtype H5N1 is widely distributed among all over the world and is very important from public health, animal health and economic point of view. The virus is excreted in high concentrations in secretions and excretions including feces. It is transmitted by direct contact or indirectly through contaminated feed and water or fecal material carried through personnel and equipments. This study was carried out to check the efficacy of different disinfectants to inactivate the H5N1 virus. Efficacy of three disinfectants including ethanol (70%), NaOH (1% and 2%), and a commercial disinfectant, D125® at different dilutions were tested in inactivating the Indian H5N1 virus in poultry feces. Efficacy of disinfectants was determined by comparing the reduction in percent infectivity of virus to embryonated chicken eggs in test group and control group. The study indicated that all three disinfectants were able to inactivate the virus but their efficiency was primarily dependent on concentrations and contact time. Out of the studied disinfectants, it can be concluded that 70% ethanol, 2% NAOH and D125® (1:32 concentration) can effectively be used in laboratory and field conditions.
INTRODUCTION
Avian Influenza virus subtype H5N1 is responsible for the ongoing panzootic among domestic poultry and other birds all over the world and has caused great economic loss in the poultry industry including India (Dubey et. al., 2008). The major concern for this subtype is its zoonotic potential that has resulted in fatalities among over half of the humans it had infected. Most of the human infections reported were the result of direct or close contact with H5N1 virus infected birds or contaminated surfaces (Claas et al., 1998). India has been encountering repeated outbreaks of H5N1 virus since 2006 (Dubey et al., 2008; Murugkar et al., 2008) particularly in Tripura, West Bengal and Assam (Nagarajan et al., 2009, Tosh et al., 2011). The H5N1 virus is highly contagious and it is excreted in high concentrations in feces and respiratory discharges (Geering et al., 1995). The disease can be transmitted by direct contact with infected migratory birds and scavenging birds like crow in native surroundings as well as indirectly through contaminated feed and water and fecal material carried through personnel and equipments (Swayne and Halvorson, 2003).
To control and eradicate the H5N1 virus during an outbreak, it is very necessary to effectively disinfect all contaminated surfaces. The H5N1 HPAI virus is placed in risk group 3 infectious agents and the laboratory worker has a potentially high risk of occupational infection. Thus, handling of HPAI virus should be done in laboratory have BSL–3 facility (OIE, 2008). The recommended personal protection procedures in the laboratory also entail use of effective disinfectants for the working area, equipment and handlers. However, the suitability of the disinfectants depends on the number of factors, viz., the dilution factor, duration of disinfectant treatment, physical and biological nature of the fomites, impact on the environment and toxic side effects etc. All the disinfectants should be evaluated for their efficacy in terms of effective concentration for minimum contact time to inactivate the virus, while developing effective disinfection protocols for control and prevention of disease outbreaks. Survivability and resistance of H5N1 virus varies for different isolates due to the continuous evolution by change in molecular structure (Stallknecht et al., 1990). Various studies has been carried out to test the sensitivity of virus for different disinfectants most of which suggests that ethanol, gluteraldehyde, chlorine, NaOH and quaternary ammonium compounds can inactivate the virus. However in most of the studies the test medium in which virus was disinfected was water or allantoic fluid. The medium in which virus is present can affect the efficacy of disinfectants (Muhammad et al., 2001). The present study was carried out taking poultry feces as a medium which are main culprits in spread of virus under field conditions. It has been reported that the stability of the Avian Influenza virus at different temperature, pH, or sensitivity for disinfectants is variable for different strains (Wanaratana et al., 2010). Hence, in the present study, the efficacy of different disinfectants in inactivating the Indian H5N1 virus in poultry feces under the simulated laboratory conditions was evaluated.
MATERIALS AND METHODS
Virus
Highly Pathogenic Avian influenza virus A/ Chicken/ India/ 151466/ 2008 (H5N1) accessed from repository of HSADL Bhopal was used in this study. The virus was an isolate from the outbreak of H5N1virus in birds in Sikkim during 2008. The virus subtype was confirmed by virus isolation in embryonated chicken eggs and identified by using tests HA, HI, RT PCR and real time PCR. The H5N1 virus was amplified in ECEs and HA titre of the seed stock allantoic fluid was found to be 29.
Calculation of EID50
The EID50 titres of the virus and spiked feces were calculated using 9–11 day old embryonated specific pathogen free (SPF) chicken eggs as per Reed and Muench (1938) protocol. The EID50 of A/Ck/Sikkim/151466/2008 (H5N1) virus was found 10–10.33/mL.
Fecal Sample
Fresh fecal sample was collected from SPF chickens maintained in the SPF unit at HSADL, Bhopal and stored at –80°C until use. The fecal sample was divided in to two parts, one part was used as such as the wet feces and another part was dried aseptically in the oven at 50ºC overnight to bring the moisture level below 20% and used as the dry feces. In order to rule out the presence of any inherent toxicity or infective agent in the fecal sample, both the dry and wet fecal samples were processed and Inoculated in Eggs and incubated at 37º C for 5 days. The embryos were found alive upto 5 days and the Haemagglutination of the harvested allantoic fluid was negative indicating that the eggs were free from any infection or any toxic chemicals.
Spiking of Virus in the Feces
The spiking of feces (both dry and wet) was done in order to ensure a final virus concentration of around 106 EID50/mL which is often shed by the infected bird in feces. Both dry and wet feces were spiked with the original virus (EID50 1010.33/mL, diluted 1:100) in the ratio of 1 part diluted virus: 1 part feces and triturated with mortar and pestle to ensure proper mixing. The spiked feces were incubated at 37º C for 30 min to ensure proper adsorption of the virus to the feces. The EID 50 of the virus–feces mixture was estimated as above and was found 106 and 106.5 /mL for dry and wet feces respectively.
Disinfectant Treatment of the Spiked Feces
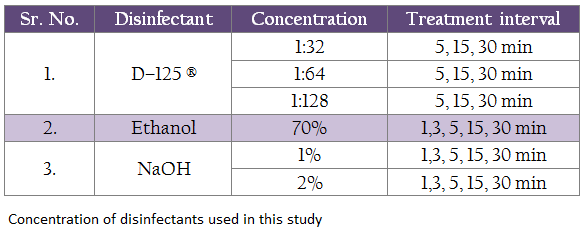
Three disinfecting agents, at various concentrations were tested individually for their ability to inactivate the virus in dry and wet feces at various time intervals as shown in Table no. I. Different dilution for all the disinfectants was prepared in the PBS. In order to rule out the presence of any inherent toxicity in the disinfectants that may kill the embryo, the disinfectants at various test dilutions were inoculated in to five embryonated chicken eggs each.
100 mg spiked feces and 100 μL of respective disinfectants respectively were taken in a 2mL screw capped tubes and mixed properly with the help of micro pestles (Tarsons, India). Virus–spiked dry and wet feces without disinfectant treatment were taken as positive control and the unspiked dry and wet feces with the disinfectants in their respective concentrations were taken as negative controls. All the tubes were incubated at room temperature for different durations as shown in the table I and were processed for virus isolation.
Virus Isolation
Isolation of virus from samples was carried out by inoculation in the embryonated chicken eggs. Before inoculation the samples were processed to remove bacterial and mycotic contaminants. After treatment with antibiotics, the samples were centrifuged and supernatant was taken for virus isolation by egg inoculation as per the protocol by WHO. Isolation of the virus was confirmed by carrying out HA test. To determine the complete inactivation of the virus, the HA negative allantoic fluids were repassaged up to third passage and tested for HA, before considering the allantoic fluids to be negative for virus isolation. The entire experiment was replicated thrice to calculate the percent infectivity and to minimize the error. The HA test was carried out as per the guidelines of OIE (OIE, 2008)
Statistical Analysis
Efficacy of different disinfection procedure was analyzed by observing the time required to completely inactivate the virus. The percent infectivity to estimate the effectiveness of the disinfectant was calculated as per the method described by Lu et al., 2003. The formula used to calculate the percent infectivity is as follow:
%Infectivity = (Infected embryos (number HA positive) × 100) / Total embryos inoculated
The percent infectivity of treated samples was compared with the control group. The reduction in percent infectivity of AIV with various disinfectants was compared with control group by using t–test (p≤0.05).
RESULTS AND DISCUSSION
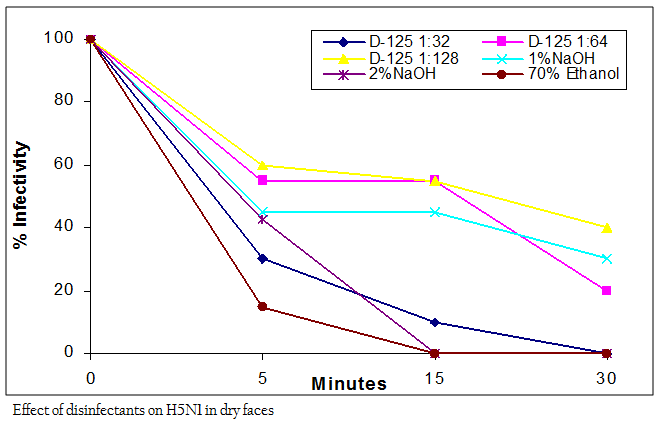
According and OIE and Government of India Action Plan, application of biosecurity measures is the first step in prevention and control of the avian influenza. Besides other logistic controls, disinfection of infected farms and premises is a very necessary step in the biocontainment process during an avian influenza outbreak (Wanaratana et al., 2010). AIVs are known to be relatively easy to be disinfected due to the lipid envelop that increases the sensitivity to dehydration, detergent and surfactants (ARCMAZ, 2000). AIV in its pure form is susceptible to 70% ethanol (Lu et al., 2003). However, ethanol may have limited effect in the
presence of organic matter and may not penetrate well into dried organic matter on surfaces. This study showed that the Indian H5N1 AIV virus was completely inactivated by 70% ethanol within 5 min in wet feces and within 15 min in dry feces. Exposure time of less than 3 min was insufficient to inactivate the virus (Table II). The results are compatible with study of Castro et al., (1998) which showed that AIV H7N2 subtype lost its infectivity only after 5 minutes in the presence of 70% Ethanol. The variation in the efficacy of ethanol to disinfect the virus in the wet and dry feces seems to be dependent on the type and consistency of the medium in which the virus is present. Sodium hydroxide (caustic soda) is a member of alkali chemicals and at 2–5% concentration has been reported to completely inactivate avian influenza viruses within 10 min It is used extensively for cleaning surfaces, especially when there is accumulated grease and tissue debris (Quinn and Markey, 2001). In this study 1% NaOH was not able to inactivate the virus in 30 min of contact time however the infectivity was reduced (Figure I and II). On the other hand, 2% NaOH was effective in completely inactivating the virus within 15 min of interaction time in both wet and dry feces (Table II). Results reported by Alphin et al. (2009) on LPAI viruses using 2% NaOH are in accordance with our findings in inactivating the virus completely on nonporous surfaces whereas 1% NaOH failed to inactivate the AIV and showed inconsistent results. This suggests that a minimum concentration of 2% of NaOH is required for reliable inactivation of AIVs and is in support to the action plan of DADF (2006) that recommended same concentration of NaOH for use as a disinfectant during avian influenza outbreaks. Its cleaning activity is related to the concentration and to the temperature at which it is used. However, NaOH is known for its corrosive and caustic properties on metals, especially aluminium, and is equally hazardous for workers particularly in higher concentration, if handled carelessly and without proper eye protection, rubber gloves and protective clothing (Jeffrey, 1995).
As per the manufacturer’s literature, commercially available disinfectant, D125® contains two quaternary ammonium compounds, namely alkyl dimethylbenzyl ammonium chloride and alkyl dimethylethylbenzyl ammonium chloride with a concentration of 2.37% each as active ingredients. The recommended dilution by the manufacturers for effective decontamination of surfaces is 1:64 (v/v). In addition to this recommended dilution, one dilution that was twice in concentration of the recommended dilution (1:32) and one that had half the recommended dilution (1:128) was tested for the efficacy of the disinfectant in the present study. At 1:32 dilution, the disinfectant inactivated the virus within 15 min in wet feces and 30 min in dry feces (Figure I and II). At 1:64 dilution, the disinfectant inactivated the virus in wet feces within 30 min, however in dry feces it failed to completely inactivate the virus within 30 min of treatment. The higher dilution of D–125® in the ratio of 1:128 was not effective (Table II). The result showed that the efficacy of the disinfectant appeared to be concentration–dependant. As the concentration of the disinfectant was increased, there was less viral recovery. Since the commercial product in the present study was based only on the quaternary ammonium compounds, its application for disinfecting the virus present in organic matter would probably be different as compared to that in the other medium.
In some previous study, the same disinfectant demonstrated relatively better efficacy against the H5N1 virus present in the chicken allantoic fluid than in poultry faeces wherein 1:64 dilution of the disinfectant was able to completely inactivate H5N1 virus within 15 min (Murugkar et al., 2008). The study suggests that use of any commercial product should be based on the field situation. Many workers have suggested the use of a cocktail of disinfectants in a single product to increase the disinfectant efficacy (Lu et al., 2003). However, any modification in the formulations without proper study could reduce the efficacy of the disinfectants and careful attention must be paid to the mixing of different products (Benedictis et al., 2007). All the tested disinfectants in this study showed an statistically significant reduction in percent infectivity of AIV as compared to control group ( p≤0.05). But there lies a difference in ability of various disinfectants to inactivate AIV with respect to time and concentration as indicated in the results of this study also substantiated by replication of the experiment thrice. The results of this study indicated that 70% ethanol for laboratory work or NaOH (2%) at the field level were effective disinfectants for inactivation of influenza viruses. NaOH should be use in the poultry farms to decontaminate poultry house but has toxicity and corrosivity issues, so it should be used cautiously (Jeffrey, 1995). However, in view of personal safety where toxicity and corrosive nature of the chemicals become the issues of greater importance, D125® in 1:32 dilutions in open areas having accumulation of dried fecal mass and 1:64 dilutions for covered areas with wet or semi–dried fecal mass would be suitable for varied environments. Virucidal activity of the majority of disinfectants is influenced by the interaction with organic material, moisture in medium and outside environment. The effect of disinfectants depends on the time of exposure as well as the environmental temperature. A previous study also reported that the disinfectants may be effective at half of the concentration when the contact time is increased (Muhammad et al. 2001). In the present study, when the concentrations of two of the disinfectants, viz., NaOH (1%) and D125® (1:128) were halved as compared to the most effective concentrations, there was a marked reduction in the efficacy of the disinfectants to inactivate the virus. All the treatments in the present studies were carried out at room temperature (24º–26ºC) which is considered optimum for effectiveness of the disinfectants (Samberg and Meroz, 1995). The moisture in the environment acts as a medium for disinfectant to reach inside the feces. This may be the reason for better efficacy of the disinfectants in wet feces than in dry feces in the present study (Table II). In India, a large proportion of poultry population is reared under free range or backyard system and the tropical climate with relatively higher temperatures favour early drying of most of the faecal material that mixes in the soil reducing. The effectiveness of the disinfectants could be reduced in such conditions. During an outbreak, extensive cleaning must be performed inside and outside all infected poultry premises including the nearby structures and those shared by personnel and equipments. Surfaces must be thoroughly wet in order to improve disinfectant activity. Wet cleaning of all the infected premises must be performed systematically from back to front and from top to bottom of the farm (Samberg and Meroz, 1995). Before the use of any disinfectant in infected areas, prior use of detergents or mild alkaline compounds with surfactant action along with water washing at high pressure will ensure more effective disinfection of the area.
CONCLUSION
Efficacy of a disinfectant under the open field conditions depends on a complex interplay of a wide variety of factors such as properties of disinfectants; characteristic of diluents; type of premises to be decontaminated; presence of organic matter; environmental temperature and humidity, mode of dispensing, etc and it would be impossible to simulate such complex environmental conditions in the laboratory, however, our study has taken into account some of the major factors affecting disinfectant efficacy and can act as a guiding principle in deciding the nature of disinfection protocol to be followed for a specific area. It is therefore imperative that all the factors mentioned above must be taken into account in order to achieve the goal of decontaminating the infected area and successfully limiting infections to uninfected farms.
ACKNOWLEDGEMENT
The authors like to express their gratitude to Joint Director, HSADL, Bhopal for providing the facilities to carry out this study. The authors would also like to acknowledge the timely help and guidance provided by all the scientific staff of HSADL Bhopal.
REFERENCES
Alphin RL, Johnson KJ, Ladman BS, Benson ER (2009). Inactivation of avian influenza virus using four common chemicals and one detergent. Poultry Sci. 88: 1181–85.
http://dx.doi.org/10.3382/ps.2008-00527
PMid:19439628
ARCMAZ (Agriculture and Resource Management council of Australia and New Zealand), (2000) Operational Procedures Manual: Decontamination. AUSVETPLAN (Australian Veterinary Emergency Plan). Department of Agriculture, Fisheries and Forestry, Canberra, Australia.
Benedictis DP, Beato MS, Capua I (2007). Inactivation of avian influenza viruses by chemical agents and physical conditions: a review. Zoonoses Public Health. 54: 51–68.
http://dx.doi.org/10.1111/j.1863-2378.2007.01029.x
PMid:17348909
Castro AE, Lu H, Senne D, Henzler D (1998). Biologic and molecular characteristics of the H7N2 subtype of avian influenza virus isolated during a 1997 field outbreak in layer flocks. In: Proceeding of the 47th Western Poultry Disease Conference, pp. 69–70.
PMid:9528722
Claas CJ, Osterhaus ADM, Beek R, De Jong J, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG (1998). Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 351: 472–77.
http://dx.doi.org/10.1016/S0140-6736(97)11212-0
DADF (2006) Action plan of Animal Husbandry for Preparedeness, control and containment of avian influenza. Published by Department of Animal Husbandry, Dairying and Fisheries, Ministry of Agriculture, Government of India. pp. 1–100.
Dubey SC, Nagarajan S, Tosh C, Bhatia S, Krishna L (2008). Avian Influenza: A long– Known Disease and its current threat. Indian J. Ani. Sci. 72: 113– 40.
Geering WA, Forman AJ, Nunn M J (1995). Exotic diseases of animals: a field guide for Australian veterinarians, Australian Government Publishing Service, Canberra.
Jeffrey DJ (1995). Chemicals used as disinfectants: active ingredients and enhancing additives. Rev. Sci. Tech. 14: 57–74.
PMid:7548972
Lu H, Castro AE, Pennick K, Liu J, Yang Q, Dunn P, Weinstock D, Henzler D (2003). Survival of avian influenza virus H7N2 in SPF chickens and their environments. Avian Dis. 47: 1015–21.
http://dx.doi.org/10.1637/0005-2086-47.s3.1015
PMid:14575104
Muhammad K, Das P, Yaqoob T, Riaz A, Manzoor R (2001). Effect of physicochemical factors on survival of avian influenza virus (H7N3 type). Int. J. Agr. Biol. 4: 416–418.
Murugkar HV, Nagarajan S, Tosh, C, Bhatia S, Venkatesh G, Jain R, Kumar S, Khandia R, Pandey M, Tripathi S, Kulkarni DD, Dubey SC (2008). H5N1 virus outbreaks in poultry in India. Vet. Rec. 162: 255.
http://dx.doi.org/10.1136/vr.162.8.255-a
PMid:18296673
Nagarajan S, Murugkar HV, Tosh C, Behera P, Jain R, Tripathi S, Khandia R, Gupta V, Kulkarni DD, Dubey SC (2009). Avian influenza virus (H5N1) in chickens in India. Vet. Rec. 164: 128.
http://dx.doi.org/10.1136/vr.164.4.128
PMid:19168888
OIE (2008) Manual of diagnosis tests and vaccines for terrestrial animals. Chapter 2.3.4. OIE, Paris, France.
Quinn PJ, Markey BK (2001). Disinfection and disease prevention in veterinary medicine. In: Block, S.S. eds., Disinfection, Sterilization, and Preservation, fifth edn. Lippincott, Williams and Wilkins: Philadelphia, PA, pp. 1069–1103.
Reed LJ, Muench H (1938). A simple method for estimating the fifty percent end points. Am. J. Hyg. 27: 493.
Samberg Y, Meroz M (1995). Application of disinfectants in poultry hatcheries. Rev. Sci. Tech. 14: 365–80.
PMid:7579636
Stallknecht DE, Shane SM, Kearney MT, Zwank PJ (1990). Persistence of avian influenza viruses in water. Avian. Dis. 34: 406–411.
http://dx.doi.org/10.2307/1591429
http://dx.doi.org/10.2307/1591428
PMid:2142420
Swayne DE, Halvorson DA (2003). Influenza. In: Saif, YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR and Swayne DE (eds.), Diseases of Poultry, 11th Edn., Iowa State Press, Ames, IA., pp. 135–160.
Tosh C, Nagarajan S, Murugkar HV, Jain R, Behera P, Katare M, Kulkarni DD and Dubey, SC (2011). Phylogenetic evidence of multiple introduction of H5N1 virus in Malda district of West Bengal, India in 2008. Vet. Microbiol. 148: 132–139
http://dx.doi.org/10.1016/j.vetmic.2010.08.015
PMid:20864277
Wanaratana S, Tantilertcharoen R, Sasipreeyajan J, Pakpinyo S (2010). The inactivation of avian influenza virus subtype H5N1 isolated from chickens in Thailand by chemical and physical treatments. Vet. Microbiol. 140: 43–48.
http://dx.doi.org/10.1016/j.vetmic.2009.07.008
PMid:19632071