Advances in Animal and Veterinary Sciences
Mini–Review Article
Advances in Animal and Veterinary Sciences 2 (4): 226 – 232Sexing of Spermatozoa in Farm Animals: a Mini Review
Mani Arul Prakash1, Arumugam Kumaresan1*, Ayyasamy Manimaran1, Rahul Kumar Joshi1, Siddhartha Shankar Layek1, Tushar Kumar Mohanty1 , Ravi Ram Divisha1
- Theriogenolology Laboratory, National Dairy Research Institute, Karnal – 132 001 Haryana
- Division of Veterinary Pharmacology, College of Veterinary Sciences, Rajendra Nagar, Hyderabad – 500 030, Andhra Pradesh
*Corresponding author: ogkumaresan@gmail.com
ARTICLE CITATION:
Prakash MA, Kumaresan A, Manimaran A, Joshi RK, Layek SS, Mohanty TK, Divisha RR (2014). Sexing of spermatozoa in farm animals: a mini review. Adv. Anim. Vet. Sci. 2 (4): 226 – 232.
Received: 2014 – 03 – 26, Revised: 2014 – 04 – 11, Accepted: 2014 – 04 – 12
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.4.226.232
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Livestock farmers always have a wish for producing young ones of desired sex. Among the several techniques available, use of sexed semen for artificial insemination is recognized as more pragmatic and easy way to pre – select the sex of the offspring. Selective use of sexed semen in breeding will not only increase the genetic progress from the daughter – dam path but would also help in producing good male germplasm from elite bulls for future breeding. Several attempts have been made, elsewhere in the globe, to develop methods that efficiently separate bovine semen into fractions containing higher concentrations of X – or Y – bearing sperm. These technologies include sex specific antibodies, centrifugation and flow cytometry. Of these attempts, the only method proven to be commercially viable is flow cytometry. However, sorting pressure, speed, electrical deviation, laser radiation all leads to membrane alteration and pre – capacitation like changes in the sorted sperm leading to reduced fertility. Despite these limitations, production of sexed semen usually followed by cryopreservation is being used commercially for cattle production. Development of the instrument for increasing the sorting rate and also purity of sorting without affecting the viability and fertility is still an active area of research. The aim of this review is to update the readers with the recent developments in sexing of spermatozoa with special reference to farm animals.
INTRODUCTION
Food and Agricultural Organization (FAO) of the United Nations estimated that the world’s food needs will increase by 100% in next 50 years and 70% of that increase will have to come from increased agricultural efficiencies and advances (Raymond et al., 2009). The demand for livestock products like meat, milk and dairy products have increased globally; to meet this demand, utilization of modern technologies to promote sustainable production of animals assumes paramount importance. Pre – sexed sperm or embryo mediated livestock production along with other genomic, proteomic and phenomics technologies offers a promising breeding strategy to meet the increased demand for food production (Rath et al., 2013). Determination of sex at the earliest stage can reduce the management cost thorough selective management of superior bulls or cows. Use of sexed semen fastens the genetic progress and allows the farm manger to increase selectively the number of heifers or steers based on the need of the farm. It also reduces calving difficulty (dystocia) in first calvers (Seidel, 2007) and reduces the replacement cost besides maintaining the biosecurity in farm. Techniques for sexing of spermatozoa has been suitably modified and are being used commercially in several countries with about 90% accuracy in cattle. The available technologies have some impediment with respect to cost of production, implementation and pregnancy rate than control sperm. Development of techniques or instruments with high sorting rate and accuracy without damaging the spermatozoa would further hasten the progress of this technology. This review is intended to put light on advances in different aspects of sperm sexing in farm animals.
BASIC PRINCIPLES OF SEX–SELECTION
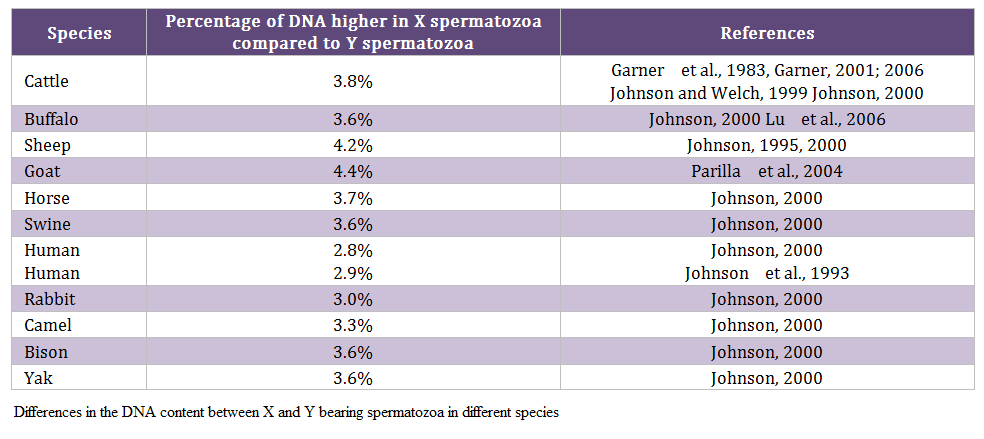
Males produce two types of spermatozoa viz X or Y, when former bearing sperm fertilizes the egg it results in formation of female and when the egg is fertilized by the Y bearing sperm it results in male offspring. Thus a pragmatic approach to sex pre – selection could be to separate the sperm population containing the desired sex and to use in artificial insemination (AI) programs. This is possible only if we realize the differences between X and Y bearing spermatozoa. The major difference between the X and Y chromosomes, reported till date, is the DNA content; the amount of DNA in X chromosome carrying spermatozoa is higher than Y chromosome carrying spermatozoa. Other differences include the size of spermatozoa i.e. X sperm is larger than Y sperm (Cui and Matthews, 1993; Cui, 1997; Moruzzi, 1979), motility (motility is reported to be higher in Y chromosome than X chromosome bearing spermatozoa) (Shettles, 1960), surface charges in sperm (X sperm has a negative charge and Y sperm has a positive charge) (Kiddy and Hafs, 1971) and cell surface antigens (Hoppe and Koo, 1984). Among these differential characteristics, differences in DNA content of spermatozoa have been shown to be the potential criteria for sorting of spermatozoa. The differences in the DNA content between X and Y bearing spermatozoa in different species and breeds are given in table 1 and 2, respectively.
METHODS OF SPERM SEXING
Albumin Gradient (or) Gradient Swim Down Procedure
This method is based on the differences between the X and Y bearing spermatozoa in the ability to swim down in a gradient solution. Since Y bearing spermatozoa are smaller in size and have high motility, they exhibit a greater downward swimming velocity than X chromosome bearing spermatozoa. Thus the fractions of semen isolated from specific part of albumin gradient are expected to be either X/Y enriched fractions. Success rate in this method has been reported to be around 75% (Ericsson et al., 1973; Beernink et al., 1993).
Percoll Density Gradient Method
This method utilizes the differences in the sedimentation density between X and Y bearing spermatozoa. Due to high sedimentation density of X bearing spermatozoa, it settles in the bottom of column while Y bearing spermatozoa remain at the top of column. Success rate in this method ranged from 86% to 94% (Lizuka et al., 1987; Van Kooij and van Oost, 1992).
Swim Up Procedure
Size – mediated difference of spermatozoa was utilized by several researchers for sperm sorting through different methods (Van Munster et al., 1999; Ollero et al., 2000). Y bearing spermatozoa are reported to swim faster than X bearing spermatozoa due its smaller size. Success rate in this method was reported to be 81% (Check et al., 1989).
/p>
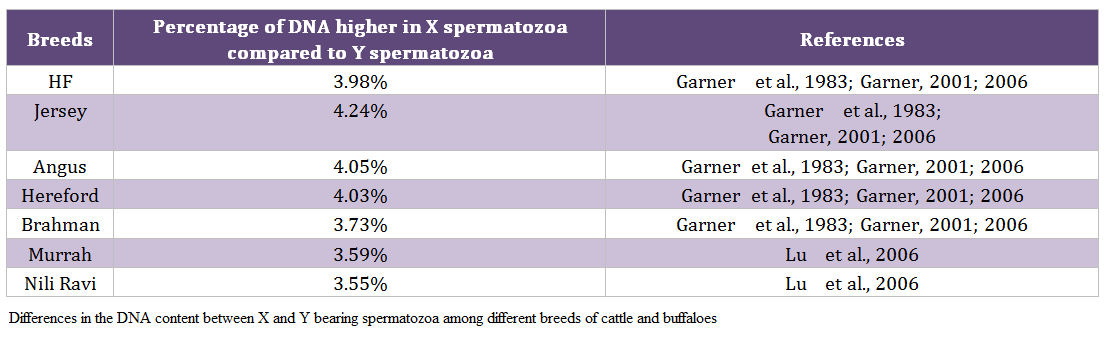
Table 2: Differences in the DNA content between X and Y bearing spermatozoa among different breeds of cattle and buffaloes
Free Flow Electrophoresis
This method is based on the presence of electric charges on the surfaces of spermatozoa. Surface of X spermatozoa are charged negative, while the surface of Y spermatozoa is charged positive. Based on electric field of separation, X and Y spermatozoa were separated using the differences in the surface charges (Kiddy and Hafs, 1971, Mohri et al., 1986, Kaneko et al., 1984).
Identification of H–Y Antigen
Identification of surface proteins expressed in either X or Y bearing spermatozoa and using immunological methods to identify and separate X and Y bearing spermatozoa could be an option. This method of sorting can be applied in large scale sperm sorting. Using Specific antibodies against H – Y antigen (expressed in Y bearing spermatozoa) sorting of spermatozoa through affinity chromatography or magnetic bead was tried with efficacy of >90% (Hoppe and Koo, 1984; Hendriksen et al., 1996; Hendriksen, 1999; Blecher et al., 1999).
Sperm Sorting Based on the Volumetric Differences
This method use image analysis of spermatozoa using interference microscopy to demonstrate a difference in sperm head volume based on the DNA content between X and Y chromosome bearing spermatozoa. A method based on this principle has been developed for sorting live spermatozoa by using interference microscopy optics with a flow cytometer. Success rate in this method has been reported to be < 80% (Van Munster et al., 1999; Van Munster, 2002).
Centrifugal Counter Current Distribution
This is a chromatographic process that partitions cells into a stationary, lower phase and a mobile, upper phase. Using this method attempts were done to sex ram spermatozoa by centrifugal counter current distribution using an aqueous two – phase system. Centrifugation was used to speed the partitioning process, so a set of 59 partitions was done in about 1 hour. Success rate in this method has been reported to be 75% (Ollero et al., 2000).
Flow Cytometry
Flow cytometers are the advanced cell sorters that use LASER to excite fluorescent dye that binds to the DNA in spermatozoa. The DNA percent and DNA specific dye are the major principle for sperm sexing through flow cytometry. In this method of sorting, the spermatozoa are treated with dye (e.g. Hoechst 33342), which is permeable to live and intact sperm membranes and binds to the DNA. Stained spermatozoa are transported to a point where they are exposed individually to a UV laser beam (wavelength of 351 – 364 nm) and the bright blue fluorescence emitted is detected and analyzed. Due to more DNA content in X chromosome bearing spermatozoa, it takes more stain than Y sperm. On the basis of this fluorescence, spermatozoa are classified as X or Y chromosome bearing and sorted. Another dye, commonly called “red quencher food colouring dye”, selectively penetrates into the damaged, dead and non – intact sperm membranes giving a red colour. Identification of live & dead sperm should be done before sorting process. Based on the excitation, spermatozoa are separated into discrete populations. In domestic animals the differences in DNA content between X and Y bearing spermatozoa ranges from 3 – 4.5% (Johnson et al., 1987; Johnson, 2000). Success rate in this method has been reported to be 85 – 95% (Pinkel et al., 1982; Johnson et al., 1989, 2000).
Among the various methods, flow cytometry based separation of sex specific spermatozoa is more popular and no other method has been consistently proven to be effective in producing offspring of the predicted sex till to date.
CONFIRMATION OF SEX SORTING ACCURACY THROUGH IMMUNOLOGICAL METHODS
Recently, polymerase chain reaction (PCR) and fluorescent in situ hybridization (FISH) techniques based sex chromosome determination has been tried in many species with differential results (Clapcote and Roder, 2005). Although PCR based detection of DNA sequences specific to X and Y chromosomes is effective for processing large numbers of samples, various limitations in these technique caused less popularity for its use in sexing spermatozoa. For example, the small amount of nuclear material in spermatozoa requires extensive rounds of amplification and contamination of samples often yields false positive results (Seidel, 1999). In FISH technique, complementary sequences on interphase X and Y chromosomes are labelled with multi – coloured DNA probes (e.g. FITC, Cy3) for visual confirmation of not only the presence but the number of X and Y chromosomes. Therefore, FISH can provide a more complete identification of sex – specific spermatozoa than PCR – based techniques. Lower rate of error in FISH techniques (3 – 7%) than PCR (8 – 23%) is further advantage of FISH (Sato et al., 2003). However, both the techniques are complexity and cost of analysis is high.
DEVELOPMENTS IN SPERM SEXING TECHNOLOGY
- First attempts to separate X and Y bearing sperms were made by Gledhill et al. (1976) through analytical flow cytometry.
- Sperm sorting technology was first developed at Lawrence Livermore National Laboratory where Pinkel et al. (1982) separated the X and O sperm nuclei of the Microtus oregoni, which have 9% DNA content difference of its sex determining chromosomes.
- Application to domestic livestock sperm separation was then implemented at Beltsville Agriculture Research Centre, USDA.
- Highly condensed sperm nucleus with unusual shape of sperm head caused difficult in quantitative fluorescence measurement and thus marginal successful in separation of sperms. Pinkel et al., (1982) overcome sperm heads associated problems through development of flow cytometry precisely for sperm sorting that orient the sperm heads with flattened side. Due to the correct orientation of sperm heads and thus precise measurements of DNA content, separation of sorted sperms was successfully done in mammalian species by Pinkel et al., (1982).
- In 1989, a major breakthrough in sperm sexing was reported by Johnson et al. (1989) through production of live offspring from sex sorted live rabbit spermatozoa after surgical insemination in the oviduct.
- Flow cytometry for sperm sexing is a patent procedure and patency lies with the M/s X – Y – INC Colorado (USA).
- Through license of sexing technology to many companies, sexed semen has been produced for nearly 18 different breeds of cattle in USA and in European countries.
TYPE OF SPERM SORTER AND SORTING EFFICIENCY
Sperm sorting through flow cytometer is dependent on the sperm head orientation towards the laser beam. Among the livestock species, bull spermatozoa has been found to be more efficiently sorted out using flow cytometry owing to its flat or oval shaped head which orient perfectly to the laser beam. Currently, by applying flow cytometer, analysis rates of about 20,000 sperm per second and a sorting speed of up to 6000 or more spermatozoa per second, each of X and Y populations, reaching purities around 90% or better, have been described. Under these conditions, approximately 15 – 20 million sorted X and Y spermatozoa could be produced per hour in most farm animal species (Hamano, 2007). The following are some of the factors that affect the sorting rate and efficiency. In the older class of sorters, the samples are sorted under 0.84 kg/cm2 of pressure with the sorting speed of 3,50,000 sperm/h (Johnson et al., 1989). The newer generation sorters are the ‘high – speed’ cell sorters and operate at sample pressures that range from 0.84 kg/cm2 to 4.22 kg/cm2. This sorting system can produce 6 million X sperm and 6 million Y sperm per hour. Greater output of 11 million spermatozoa/h has been reported by sorting only X sperm with 85 – 90% purity (Johnson and Welch, 1999). Original sperm sorter developed by M/s USDA Beltsville utilized the beveled injection needle within the sorting nozzle of an ortho flow cytometer. Subsequently, the high speed flow cytometer was developed at Lawrence Livermore National Laboratory by Van den Engh and Stokdijk, (1989) and commercialized as MOFLO TM cytometer. Both Ortho and high speed flow cytometer has beveled needle in the nozzle. But the fluidic orientation rate was high in high speed flow cytometer. In the Modified flow cytometer (MOFLO) system fluidic orientation rate exceeding 20,000 spermatozoa per sec and sorting rate up to 60,000 or more sperms per second for each of X and Y spermatozoa at 90% accuracy. Additional 2 pmt incorporated at 45˚ and 135˚ relative to 0˚ detector, increased orientation efficiency by 5 – 15% (Sharpe and Evans, 2009). Latest advancement in flow cytometry is dual headed flow cytometry.
SPERM DEFECTS DUE TO SORTING PROCEDURES
Dye, sorting speed, pressure, laser light, electrical charging and deviation and changes in the medium collectively leads to defects in spermatozoa.
Dye Defects
Addition of DNA specific dye (Hoechst 33342) causes the chromatin decondensation (Johnson and Seidel, 1999). Among farm animals, Boar has the stable chromatin compared to other farm animals (Bathgate, 2008). However, dye – mediated disturbances of heat shock proteins HSP70 and capacitation like changes in the sperm membranes has also been reported in boar sperms (Spinaci et al., 2006).
Sorting Pressure and Speed
High sorting pressure of 40 – 60 psi and high speed (55 – 60 mph) makes the sperm more vulnerable for the damage of DNA during sorting (Suh et al., 2005).
U–V Laser
Adverse effects of UV rays on DNA integrity is well known phenomenon. Laser power of 200 MW or higher had detrimental effect on the fertilizing ability of the rabbit sperm due to destruction of chromatin integrity than sperm exposed to 125 MW (Silva and Gadella, 2006).
Electrical Charging and Electrical Deviation
Due to electrical charging and electrical deviation the sperm membranes of mid piece and tail undergoes depolarization. Further, relative oxygen species produced by the electrical forces, reduced mitochondrial activity of sperm (Rath and Johnson, 2008). Stressors due to sorting process may damage the DNA to some extent which may potentially compete with spermatozoa having normal DNA and reduce embryonic viability (Tesarik et al., 2004).
Changes in Medium
Changes in pH and osmolarity during sorting process decrease the sperm fertilizing ability (Gadella and Harrison 2000; Harrison and Gadella, 2005). Although the sperm is normally exposed to different pH milieu in female reproductive tract to achieve fertilization, changes of pH during sorting process affects the fertilizing ability of sorted sperm. Any alteration in the sperm physiology like modification of membrane stability, sperm motility or acrosome homeostasis, has a direct impact on the fertilizing capacity of sperm while the altered DNA quality affects the embryo quality leads to syngamy after fertilization of gametes. Removal of seminal plasma, sorting pressure, speed, electrical deviation, laser radiation all leads to membrane alteration (depolarization) and pre – capacitation like changes in the sorted sperm (Vazquez et al., 2003; Seidel and Schenk, 2008; Schenk et al., 2009). Further, alteration of membrane proteinase due to sorting and freezing were reported by de Graaf et al., (2008) and Spinaci et al., (2005). Overall, it is accepted that the sorting procedures reduce the life span of spermatozoa. Shorter life span cause reduced motility and thus reduced fertility of sex sorted spermatozoa (Rath et al., 2003; Maxwell et al., 2004; Peippo et al., 2009).
Post Sorting Centrifugation and Cryo–Preservation
Since less number of spermatozoa are available after sorting, generally centrifugation is done to concentrate the spermatozoa in small volume. Centrifugation of sorted sperms cause further damage in already stressed sperm and impairs the fertilizing ability of sorted sexed spermatozoa (Maxwell et al., 1999) and decreases the life span of spermatozoa (Sa’ Filho et al., 2010). Staining and centrifugation of sorted spermatozoa increased the percentage of dead and damaged spermatozoa by 18.6% (Garner and Johnson, 1995). Cryopreservation of sorted semen includes dilution, cooling, freezing and thawing which further leads to damage of plasma membrane (Underwood et al., 2010).
MEASURES TO REDUCE THE DEFECTS IN SPERMATOZOA DURING SORTING
- Lowering the sorting pressure from standard pressure of 50 psi to 40 psi improved sorted spermatozoa quality without significant decrease in sorting efficiency in bull and stallion (Suh et al., 2005).
- UV laser with argon or solid state laser has been shown to reduce the defects associated with sperm membranes and DNA (Rath and Johnson, 2008).
- Addition of seminal plasma (10% v/v) into the staining medium has been shown to improve the viability, motility and reduce capacitation like changes in boar and ram spermatozoa (de Graaf et al., 2008) as it act as inhibitor of capacitation and maintains pH as alkalinity of spermatozoa in female reproductive tract.
- Addition of bovine sheath fluid (197mM tris, 55.4mM citric acid, 47.5mM fructose) in the extender and addition of protamine before sorting process improved the sperm viability, motility and maintains the fertility of sperms (Gosalvez et al., 2011).
- Gradient centrifugation prior to sperm sexing also improved the resolution and sorting rates.
- Extensive research is being carried out to reduce the sperm defects due to sorting process. Use of impermeable dyes or permeable dyes at low concentration could be an option to reduce the dye induced defects in sorted spermatozoa.
PREGNANCY RATE WITH SEXED SEMEN
In 1989, a major breakthrough in sperm sexing was achieved through production of live offspring’s from sex sorted rabbit sperm by Johnson et al (1989). Subsequently, hundreds of pre – sexed calves were born as a result of AI (Seidel, 2007). The first dairy calf from pre – determined sex after AI was reported by Seidel et al (1997) and first preselected calf was born through AI with frozen semen in 1999. The birth of the first calf after transfer of embryos produced following in – vitro fertilization with frozen thawed, sex sorted sperm was reported by Puglisi et al (2006).
Conception rate with sexed semen in heifers was about 70 – 80% and in lactating cows was about 50 – 60% (Seidel, 2003). In another study, it was reported that the conception rate in heifers was 45% and in lactating cows was 28% (De Vries et al., 2008). In contrast, conception rate of 69.7% with sexed sperm & 66.5% with unsexed sperm following AI was reported in China (Lu et al., 2010). In general, conception rate in lactating animals is low due to low insemination dose, large postpartum uterus and weak heat symptoms (Yoshida and Nakao, 2005). Therefore, higher number of inseminations and insemination doses are required for lactating cows to achieve more conception rate (Peppio et al., 2009).
LIMITATION OF SEXED SEMEN TECHNOLOGY
Limitations can be due to different stages of processing level, staring from equipment, semen sorting procedure, post – sorting procedures, techniques of insemination and fertilizing ability of sexed semen.
- Cost of equipment and its patented technology are high.
- High cost for maintenance.
- Require skilled manpower for operation and supervision of machine.
- Slow process. i.e. less number of spermatozoa sorted per hour due to sexing of one sperm at a time rather than multiple sperm and thus less number of sperm are being identified for its sex or only less number of straws are being produced (7 – 10 dose/hour) (Seidel, 2007).
- Half of sperm sample are unsexable and go as waste (only 30% of sperm are sexable in which only 15% responsible for female offspring), leading to increased cost of sexed semen compared to unsexed semen ($35 – 65 vs $15 – 20).
- Efficiency of sexing of sperm is best with fresh sperm, so sorters should be located near the bull’s stations.
CONCLUSIONS
Success of sexed semen industry depends upon the sorting speed, accuracy and the fertility of sorted spermatozoa. The existing technologies, although used at commercial scales, are to be further refined for mass scale use of sexed semen. As on date sex fixing of sperms or sex sorting through flow cytometer is the only fully validated method for sperm sexing. Although, it has more constraints on sexing sperms procedures and sexed semen quality (life span, spermiogram, fertilizing ability) several improvements have been recently made in sperm sorting procedure and less harmful for sperms. In future, it is possible to improve the existing methods or to develop entirely a new technology package for sorting spermatozoa. Identification of sperm surface markers specific for X or Y bearing spermatozoa and using them to sort spermatozoa is an option. Targeted killing i.e. killing of unwanted sex bearing spermatozoa either at the production site itself or after ejaculation could also be an option. Developing designer bulls that produce only one type (either X or Y) of spermatozoa by knocking out the other type is also possible, however it requires intensive research. Whatever technologies we use, it all depend on the good management practices to achieve high conception or pregnancy rate with the predicted sex from the sorted sexed semen.
REFERENCES
Bathgate R, (2008). Functional integrity of sex – sorted, frozen – thawed boar sperm and its potential for artificial insemination. Theriogenol. 70: 1234 – 1241.
http://dx.doi.org/10.1016/j.theriogenology.2008.06.009
PMid:18640713
Beernink FJ, Dmowski WP, Ericsson RJ (1993). Sex pre selection through albumin separation of sperm. Fertil Steril. 59: 382 – 386.
PMid:8425635
Blecher SR, Howie R, Li S, Detmar J, Blahut L (1999). A new approach to immunological sexing of sperm. Theriogenol. 52: 1309 – 1321.
http://dx.doi.org/10.1016/S0093-691X(99)00219-8
Check JH, Shanis BS, Cooper SO, Bollendorf A (1989). Male sex preselection: swim – up technique and insemination of women after ovulation induction. Arch. Androl. 23: 165 – 166.
http://dx.doi.org/10.3109/01485018908986839
PMid:2511816
Clapcote SJ, Roder JC (2005). Simplex PCR assay for sex determination in mice. Biotechniques. 38: 702 – 706.
http://dx.doi.org/10.2144/05385BM05
PMid:15945368
Cui KH, Matthews CD (1993). X larger than Y. Nature. 366: 117 – 118.
http://dx.doi.org/10.1038/366117b0
PMid:8232551
Cui KH (1997). Size difference between human X and Y spermatozoa and Pre – fertilization diagnosis. Mol. Human Reprod. 3: 61 – 67.
http://dx.doi.org/10.1093/molehr/3.1.61
PMid:9239709
de Graaf SP, Leahy T, Marti J, Evans G, Maxwell WMC (2008). Application of seminal plasma in sex – sorting and sperm cryopreservation. Theriogenol. 70: 1360 – 1363.
http://dx.doi.org/10.1016/j.theriogenology.2008.07.012
PMid:18762331
De Vries A, Overton M, Fetrow J, Leslie K, Eicker S, Rogers G (2008). Exploring the impact of sexed semen on the structure of the dairy industry. J. Dairy Sci. 91: 847 – 856.
http://dx.doi.org/10.3168/jds.2007-0536
PMid:18218773
Ericsson RJ, Langevin CN, Nishino (1973). M. Isolation of fractions rich in human Y sperm. Nature. 246: 421 – 424.
http://dx.doi.org/10.1038/246421a0
PMid:4587152
Gadella BM, Harrison RA (2000). The capacitating agent bicarbonate induces protein kinase A – dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development. 127: 2407 – 2420.
PMid:10804182
Garner DL, Johnson LA (1995). Viability assessment of mammalian sperm using SYBR – 14 and propidium iodide. Biol. Reprod. 53: 276 – 284.
http://dx.doi.org/10.1095/biolreprod53.2.276
PMid:7492679
Garner DL (2001). Sex – sorting mammalian sperm: concept to application in animals. J. Androl. 22: 519 – 526.
PMid:11451346
Garner DL (2006). Flow cytometric sexing of mammalian sperm. Theriogenol. 65: 943 – 957.
http://dx.doi.org/10.1016/j.theriogenology.2005.09.009
PMid:16242764
Garner DL, Gledhill BL, Pinkel D, Lake S, Stephenson D, Van Dilla MA (1983). Quantification of the X – and Y – chromosome bearing sperm of domestic animals by flow cytometry. Biol. Reprod. 28: 312 – 321.
http://dx.doi.org/10.1095/biolreprod28.2.312
PMid:6682341
Gledhill BL, Lake S, Steinmetz LL, Gray JW, Crawford JR, Dean PN (1976). Flow microfluorometric analysis of sperm DNA content: effect of cell shape on the fluorescence distribution. J. Cell Physiol. 87: 367 – 376.
http://dx.doi.org/10.1002/jcp.1040870312
PMid:56337
Gosálvez J, Ramirez MA, López – Fernández C, Crespo F, Evans KM, Kjelland ME, Moreno JF (2011). Sex – sorted bovine spermatozoa and DNA damage: I Static features. Theriogenol. 75: 197 – 205.
http://dx.doi.org/10.1016/j.theriogenology.2010.08.006
http://dx.doi.org/10.1016/j.theriogenology.2010.09.011
PMid:21040960
Hamano K (2007). Sex Preselection in Bovine by separation of X and Y chromosome bearing spermatozoa. J. Reprod. Dev. 53: 27 – 38
http://dx.doi.org/10.1262/jrd.18141
PMid:17332697
Harrison RA, Gadella BM (2005). Bicarbonate – induced membrane processing in sperm capacitation. Theriogenol. 63: 342 – 351.
http://dx.doi.org/10.1016/j.theriogenology.2004.09.016
PMid:15626403
Hendriksen PJM (1999). Do X and Y spermatozoa differ in proteins? Theriogenol. 52: 1295 – 1307.
http://dx.doi.org/10.1016/S0093-691X(99)00218-6
Hendriksen PJM, Welch GR, Grootegoed JA, VanderLende T, Johnson, LA (1996). Comparison of detergent – solubilized membrane and soluble proteins from flow cytometrically sorted X – and Y – chromosome bearing porcine spermatozoa by high resolution 2 – D electrophoresis. Mol. Reprod. Dev. 45: 342 – 450.
http://dx.doi.org/10.1002/(SICI)1098-2795(199611)45:3<342::AID-MRD11>3.0.CO;2-0
http://dx.doi.org/10.1002/(SICI)1098-2795(199611)45:3<342::AID-MRD11>3.3.CO;2-S
Hoppe PC, Koo GC (1984). Reacting mouse sperm with monoclonal H – Y antibodies does not influence sex ratio of eggs fertilized in vitro. J. Reprod. Immunol. 6: 1 – 9.
http://dx.doi.org/10.1016/0165-0378(84)90036-6
Johnson LA, Seidel Jr GE (1999). Current status of sexing mammalian sperm. Theriogenol. 52: 1267 – 1484.
http://dx.doi.org/10.1016/S0093-691X(99)00215-0
Johnson LA, Welch GR (1999). Sex preselection: High – speed flow cytometric sorting of X and Y sperm for maximum efficiency. Theriogenolol. 52: 1323 – 1341.
http://dx.doi.org/10.1016/S0093-691X(99)00220-4
Johnson LA (1995). Separation of X and Y chromosome bearing sperm based on DNA differences. Reprod. Fertil. Dev. 7: 893 – 903.
http://dx.doi.org/10.1071/RD9950893
PMid:8711222
Johnson LA (2000). Sexing mammalian sperm for production of offspring: the state – of – the art. Anim. Reprod. Sci. 60 – 61: 93 – 107
Johnson LA, Flook JP, Hawk HW (1989). Sex preselection in rabbits: live births from X and Y sperm separated by DNA and cell sorting. Biol. Reprod. 41: 199 – 203.
http://dx.doi.org/10.1095/biolreprod41.2.199
PMid:2804212
Johnson LA, Flook JR, Look MV (1987). Flow cytometry of X and Y chromosome – bearing sperm for DNA using an improved preparation method and staining with Hoechst 33342. Gamete. Res. 17: 203 – 212.
http://dx.doi.org/10.1002/mrd.1120170303
PMid:3507347
Johnson LA, Welch GR, Keyvanfar K (1993). Gender preselection in humans? How cytometric separation of X and Y spermatozoa for the prevention of X – linked diseases. Hum. Reprod. 8: 1733 – 1739.
PMid:8300839
Kaneko S, Oshiro S, Kobayashi T, Itzuka R, Mohri H (1984). Human X – and Y – bearing sperm differ in cell surface sialic acid content. Biochem. Biophys. Res. Commun. 124: 950 – 955.
http://dx.doi.org/10.1016/0006-291X(84)91050-7
Kiddy CA, Hafs HD (1971). Sex ratio at birth—prospects for control. Am. Soc. Anim. Sci. 4: 104.
Lizuka R, Kaneko S, Aoki R, Kobayashi T (1987). Sexing of human sperm by discontinuous Percoll density gradient and its clinical application. Hum. Reprod. 2: 573 – 575.
Lu YQ, Zhanga M, Lu SS, Xu D, Huang W, Menga B, Xua H, Lu KH (2010). Sex – preselected buffalo (Bubalus bubalis) calves derived from artificial insemination with sexed sperm. Anim. Reprod. Sci. 119: 169 – 171.
http://dx.doi.org/10.1016/j.anireprosci.2010.01.001
PMid:20137870
Lu YQ, Zhang M, Meng B, Lu SS, Wei YM, Lu, KH (2006). Identification of X – and Y – chromosome bearing buffalo (Bubalus bubalis) sperm. Anim. Reprod. Sci. 95: 158 – 164.
http://dx.doi.org/10.1016/j.anireprosci.2005.11.005
PMid:16413705
Maxwell WMC, Evans G, Hollinshead FK, Bathgate R, De Graaf, SP, Eriksson BM (2004). Integration of sperm sexing technology into the ART toolbox. Anim. Reprod. Sci. 82 – 83: 79 – 95.
Maxwell WMC, Evans G, Mortimer ST, Gillan L, Gellatly ES, McPhie CA (1999). Normal fertility in ewes after cervical insemination with frozen – thawed spermatozoa supplemented with seminal plasma. Reprod. Fertil. Dev. 11: 123 – 126.
http://dx.doi.org/10.1071/RD99046
PMid:10735556
Mohri H, Oshio S, Kaneko S, Kobayashi T, Lizuka R (1986). Separation and characterization of mammalian X – and Y – bearing sperm. Dev. Growth Diff. 28 (1): 35 – 36.
http://dx.doi.org/10.1111/j.1440-169X.1986.28s1_35.x
Moruzzi JF (1979). Selecting a mammalian species for the separation of X – and Y – chromosome – bearing spermatozoa. Reprod. Fertil. 57: 319 – 323.
http://dx.doi.org/10.1530/jrf.0.0570319
Ollero M, Perez – Pe R, Gargallo I, Morlanes S, Osada J, Mui-o – Blanco T, Cebrian – Perez J (2000). Separation of ram spermatozoa bearing X and Y chromosome by centrifugal counter current distribution in an aqueous two – phase system. J. Androl. 21: 921 – 928.
PMid:11105919
Parrilla I, Vazquez JM, Roca J, Martinez EA (2004). Flow cytometry identification of X – and Y – chromosome bearing goat sperm. Reprod. Dom. Anim. 39: 58 – 60.
http://dx.doi.org/10.1046/j.1439-0531.2003.00480.x
PMid:15129923
Peippo J, Vartia K, Kananen – Anttila K, R aty M, Korhonen K, Hurme T, Myllymäki H, Sairanen A, Mäki – Tanila A (2009). Embryo production from superovulated Holstein – Friesian dairy heifers and cows after insemination with frozen – thawed sex – sorted X spermatozoa or unsorted semen. Anim. Reprod. Sci. 111: 80 – 92.
http://dx.doi.org/10.1016/j.anireprosci.2008.02.002
PMid:18359583
Pinkel D, Gledhill BL, Lake S, Stephenson D, Van Dilla MA (1982). Sex pre-selection in mammals? Separation of sperm bearing Y and ''O'' chromosomes in the vole Microtus oregoni. Sci. 218: 904 – 905.
http://dx.doi.org/10.1126/science.6753153
Puglisi R, Vanni R, Galli A, Balduzzi D, Parati K, Bongioni G (2006). In vitro fertilization with frozen – thawed bovine sperm sexed by flow cytometry and validated for accuracy by realtime PCR. Reprod. 132: 519 – 526.
http://dx.doi.org/10.1530/rep.1.01173
PMid:16940293
Rath D, Johnson LA (2008). Application and commercialization of flow cytometrically sex – sorted semen. Reprod. Dom. Anim. 43(2): 338 – 346.
http://dx.doi.org/10.1111/j.1439-0531.2008.01182.x
PMid:18638144
Rath D, Barcikowski S, De Graaf SP, Garrels W, Grossfeld R, Klein S, Knabe W, Knorr C, Kues W, Meyer H, Michl J, Moench – Tegeder G, Rehbock C, Taylor U, Washausen S (2013). Sex selection of sperm in farm animals: status report and developmental prospects. Reprod. 1470 – 1626: 1741 – 7899.
Rath D, Ruiz S, Sieg B (2003). Birth of female piglets following intrauterine insemination of a sow using flow cytometrically sexed boar semen. Vet Rec. 152: 400 – 401.
http://dx.doi.org/10.1136/vr.152.13.400
PMid:12696707
Raymond R, Bales CW, Bauman DE, Clemmons D, Kleinman R, Lanna D, Nickerson S, Sejrsen K (2009). Recombinant Bovine somatotropin (rbST): A safety assessment. ADSA – CSAS – ASAS; Joint Annual Meeting. Montreal, Canada.
Sá Filho MF, Ayres H, Ferreira RM, Nichi M, Fosado M, Filho CEP, Baruselli PS (2010). Strategies to improve pregnancy per insemination using sex – sorted semen in dairy heifers detected in estrus. Theriogenol. 74: 1636 – 1642.
http://dx.doi.org/10.1016/j.theriogenology.2010.06.036
PMid:20833421
Sato T, Ikuta K, Sherlock J, Adinolfi M, Suzumori K (2003). Comparison between fluorescence in situ hybridization (FISH) and quantitative – fluorescent polymerase chain reaction (QF – PCR) for the detection of aneuploidies in single blastomeres. Prenat Diagn. 23: 678 – 684.
http://dx.doi.org/10.1002/pd.660
PMid:12913875
Schenk JL, Cran DG, Everett RW, Seidel GE Jr (2009). Pregnancy rates in heifers and cows with cryopreserved sexed sperm: Effects of sperm numbers per inseminate, sorting pressure and sperm storage before sorting. Theriogenol. 71: 717 – 728.
http://dx.doi.org/10.1016/j.theriogenology.2008.08.016
PMid:19150124
Seidel GE Jr, Schenk JL (2008). Pregnancy rates in cattle with cryopreserved sexed sperm: Effects of sperm numbers per inseminate and site of sperm deposition. Anim. Reprod. Sci. 105: 129 – 138.
http://dx.doi.org/10.1016/j.anireprosci.2007.11.015
PMid:18178041
Seidel GE Jr, Allen CH, Johnson LA, Holland MD, Brink Z and Welch GR (1997). Uterine horn insemination of heifers with very low numbers of non – frozen and sexed sperm. Theriogenol. 48: 1255 – 1264.
http://dx.doi.org/10.1016/S0093-691X(97)00368-3
Seidel Jr GE (1999). Sexing mammalian spermatozoa and embryos – state of the art. J. Reprod. Fertil. Suppl. 54: 477 – 487.
PMid:10692877
Seidel Jr GE (2003). Economics of selecting for sex: the most important genetic trait. Theriogenol. 59: 585 – 598.
http://dx.doi.org/10.1016/S0093-691X(02)01242-6
Seidel Jr GE (2007). Overview of sexing sperm. Theriogenol. 68: 443 – 446.
http://dx.doi.org/10.1016/j.theriogenology.2007.04.005
PMid:17512976
Sharpe JC, Evans KM (2009). Advances in flow cytometry for sperm sexing. Theriogenol. 71: 4 – 10.
http://dx.doi.org/10.1016/j.theriogenology.2008.09.021
PMid:18950849
Shettles LB (1960). Nuclear morphology of human spermatozoa. Nature. 186: 648 – 649.
http://dx.doi.org/10.1038/186648a0
PMid:14445926
Silva PFN, Gadella BM (2006). Detection of damage in mammalian sperm cells. Theriogenol. 65: 958 – 978.
http://dx.doi.org/10.1016/j.theriogenology.2005.09.010
PMid:16242762
Spinaci M, Volpe S, Bernardini C, Ambrogi MD, Tamanini C, Seren E, Galeati, G (2005). Immunolocalization of heat shock protein 70 (Hsp70) in boar spermatozoa and its role during fertilization. Mol. Reprod. Dev. 72: 534 – 541.
http://dx.doi.org/10.1002/mrd.20367
PMid:16142794
Spinaci M, Volpe S, Bernardini C, De Ambrogi M, Tamanini C, Seren E (2006). Sperm sorting procedure induces a redistribution of Hsp70 but not Hsp60 and Hsp90 in boar spermatozoa. J. Androl. 27: 899 – 907.
http://dx.doi.org/10.2164/jandrol.106.001008
PMid:16870948
Suh TK, Schenk JL, Seidel GEJ (2005). High pressure flow cytometric sorting damages sperm. Theriogenol. 64: 1035 – 1048.
http://dx.doi.org/10.1016/j.theriogenology.2005.02.002
PMid:16125550
Tesarik J, Greco E, Mendoza C (2004). Later, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum. Reprod. 19: 611 – 615.
http://dx.doi.org/10.1093/humrep/deh127
PMid:14998960
Underwood SL, Bathgate R, Ebsworth M, Maxwell WMC, Evans G (2010). Pregnancy loss in heifers after artificial insemination with frozen – thawed, sex – sorted, re – frozen – thawed dairy bull sperm. Anim. Reprod. Sci. 118(1): 7 – 12.
http://dx.doi.org/10.1016/j.anireprosci.2009.06.004
PMid:19595524
Van den Engh G, Stokdijk W (1989). Parallel processing data acquisition system for multi laser flow cytometry and cell sorting. Cytometry. 10: 282 – 293.
http://dx.doi.org/10.1002/cyto.990100307
PMid:2714112
Van Kooij RJ, Van Oost BA (1992). Determination of sex ratio of spermatozoa with a deoxynbonucleic acid – probe and quinacrine staining: a comparison. Fertil. Sterii. 58: 384 – 386.
PMid:1378794
Van Munster EB (2002). Interferometry in flow to sort unstained X – and Y – chromosome bearing bull spermatozoa. Cytometry. 47: 192 – 199.
http://dx.doi.org/10.1002/cyto.10064
PMid:11891724
Van Munster EB, Stap J, Hoebe R, Te Meerman GJ, Aten JA (1999). Difference in volume of X – and Y – chromosome bearing bovine sperm heads matches differences in DNA content. Cytometry. 35: 125 – 128.
http://dx.doi.org/10.1002/(SICI)1097-0320(19990201)35:2<125::AID-CYTO3>3.0.CO;2-H
Vazquez JM, Martinez EA, Parrilla I, Roca J, Gil MA, Vazquez JL (2003). Birth of piglets after deep intrauterine insemination with flow cytometrically sorted boar spermatozoa. Theriogenol. 59: 1605 – 1614.
http://dx.doi.org/10.1016/S0093-691X(02)01198-6
Yoshida C, Nakao T (2005). Some characteristics of primary and secondary estrous signs in high – producing dairy cows. Reprod. Dom. Anim. 40: 150 – 155.
http://dx.doi.org/10.1111/j.1439-0531.2005.00572.x
PMid:15819966