Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (4): 222 – 225Effect of Nicotine on Hematology, Lipid Profile and Liver Enzymes in Adult Male Mice (Mus Musculus)
Saima Sharif1*, Tasnim Farasat1, Nargis Fatima1, Ayesha Farooq1, Shagufta Naz1,
- Department of Zoology, Lahore College for Women University, Jail Road, Lahore, Pakistan
*Corresponding author: ssharif1978@yahoo.com
ARTICLE CITATION:
Sharif S, Farasat T, Fatima N, Farooq A, Naz S (2014). Effect of nicotine on hematology, lipid profile and liver enzymes in adult male mice (mus musculus). Adv. Anim. Vet. Sci. 2 (4): 222 – 225.
Received: 2014–02–27, Revised: 2014–03–26, Accepted: 2014–03–27
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.4.222.225
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
This study was designed to observe the effects of nicotine on various hematological parameters, lipid and hepatic profile of adult male mice. A total of 80 mice were divided into two groups; control (n=40) and experimental (n=40). Subcutaneous injections of 1 mg/kg body weight of nicotine were given to the experimental and saline solution was given to the control group. The treatment lasted for 6 weeks after which blood was collected. Using hematological analyzer, hematological parameters were assessed whereas biochemical analysis was performed by chemistry analyzer. Statistical analysis was performed on SPSS. Comparison between the groups was done by T–test. The white blood cells, Platelet, Hematocrit, mean corpuscular volume, cholesterol, triglycerides and low density lipoprotein cholesterol were found significantly higher (p≤0.05) in an experimental group compared to control group while the significant decrease was observed in red blood cell count, hemoglobin, mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration, high density lipoprotein cholesterol and HDL–C/LDL–C ratio in the experimental group. Significant increase in the liver enzymes, alanine amino transferase aspartate aminotransferase and alkaline phosphatase were observed, whereas total bilirubin (mg/dl), albumin and total protein showed significant decrease in the experimental group. It is concluded that nicotine administration in mice resulted in deleterious effects on various hematological and biochemical parameters.
INTRODUCTION
Nicotine is thought to be the main component present in the smoke of tobacco, which works as a neuro–toxic and largely accounts for most of the deleterious effects (Slotkin, 2004). Various studies have been performed on humans, animals and in a number of various types of cell systems to largely examine the actions of nicotine (Pausova et al., 2003; Cooke & Bitterman, 2004; Valenca et al., 2004).
Changes in the hematological parameters due to the inhalation of nicotine may be an important reason for various vascular diseases. Inhalation of considerable concentration of nicotine cause alternations in various hematological parameters, including white blood cells, mean corpuscular volume, hematocrit, hemoglobin, monocyte, eosinophil, neutrophil and lymphocyte counts (Bain et al., 1992). Liver is the major site of nicotine metabolism and the metabolites of nicotine are immersed in the liver. Elevations of alkaline phosphates are the symptoms of diseases of the liver. (Snyder et al., 1993; Neurath, 1994).
As the genetic knowledge of mice grow rapidly far beyond than that of rats, mouse models are becoming more prevalent in the scientific literature and for this reason mice were chosen to conduct various experiments to study the pharmacological effects of nicotine (Benatar, 2007; Gawrylewski, 2007). Hence, the main aim of the present study was to study the influence of nicotine on various hematological parameters, lipid profile and liver enzymes in adult albino mice.
MATERIALS AND METHODS
Animals
A total of 85 adult male albino mice of age one month old were collected from Veterinary Research Institute (VRI) Lahore with the body weight range from 19–27(g). The Animals Ethical Committee of Lahore College for Women University approved the animal studies. The animals were kept at animal house of Lahore College for Women University, Lahore and fed on standard pelleted diet and tap water.
Experimental Design
The animals were acclimatized for a period of one month prior to experiment in animal house of Lahore College for Women University, Lahore. During acclimatizing period 5 mice died. To decrease the effects of circadian rhythm all treatments were given between 9 to 10 a.m. These animals were kept in wire cages in groups of 20 animals per cage. All the animals were kept under similar management and feeding conditions throughout the experiment and 12–h light/12–h dark cycle with ambient temperature of 20–22ºC was maintained. The relative humidity was 60%. After acclimatizing period mice were indiscriminately distributed into two groups, control group (n=40 without nicotine treatment) and experimental group (n=40 nicotine treated (1 mg/ kg of body weight). By giving nicotine injections subcutaneously mortality was not arisen within the 6 weeks of experimental period.
Mode of Treatment
The body weight of each adult male mice was measured before giving them injections on the first day of experiment. An average weight of 26.5g was obtained. First group containing 40 mice was nominated as control in which 0.1ml of normal saline was administered subcutaneously. Second group containing 40 mice was given effective dose of 1 mg/kg of body weight of nicotine hydrogen tartarate subcutaneously injected in the scruff of the neck daily for 6 weeks at 10 a.m. every day. Daily weighed feed was provided early morning and then on next day the remaining feed was weighed again per cage to determine the amount of feed consumed.
Sample Collection
The blood samples were collected by performing cardiac puncture directly from the ventricle of the heart after anesthetizing the animal. To avoid hemolysis, sampling is performed with moderate suction. For hematological analysis blood is collected in EDTA coated tubes and for the assessment of lipid profile and liver enzymes serum was separated by centrifugation at 5000 rpm for 10 minutes and stored in eppendorfs at –20 ºC till analyzed.
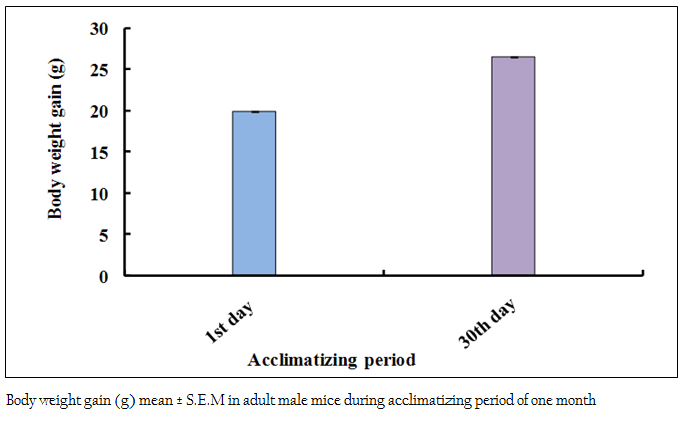
Figure 1: Body weight gain (g) mean ± S.E.M in adult male mice during acclimatizing period of one month
Assessment of Hematological Parameters
For this purpose the blood was immediately collected in ETDA tubes and the samples were immediately run on hematological analyzer (Model “KX–21 Sysmex”, Germany) to assess various hematological parameters like WBC count, RBC count, Hgb concentration, Hct, MCV, MCH, MCHC and PLT.
Assessment of Biochemical Parameters
The lipid components such as total cholesterol, LDL–C, HDL–C, triglyceride, the liver enzymes (AST, ALT and ALP) and other biochemical parameters bilirubin, total proteins, and albumin in serum were determined by using chemistry analyzer.
Statistical Analysis
Values of various hematological parameters TC, HDL–C, LDL–C, AST, ALT, ALP, triglycerides, bilirubin, albumin and total protein were given as mean ± Standard Error Mean (S.E.M). The comparisons between the parameters of control group and experimental group were statistically analyzed by using Independent Sample Student “t” Test. All the statements of significance are based on the 0.05 level of probability at 95% confidence interval. All graphs were obtained on Microsoft Excel Starter 2010 and statistical analysis was done on SPSS Version 13.0.
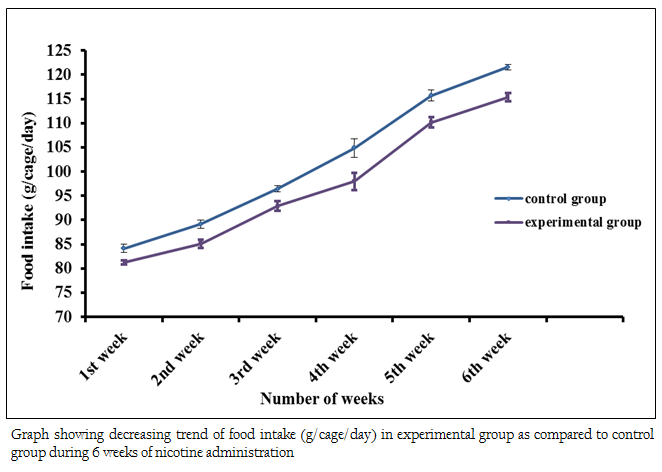
Figure 2: Graph showing decreasing trend of food intake (g/cage/day) in experimental group as compared to control group during 6 weeks of nicotine administration
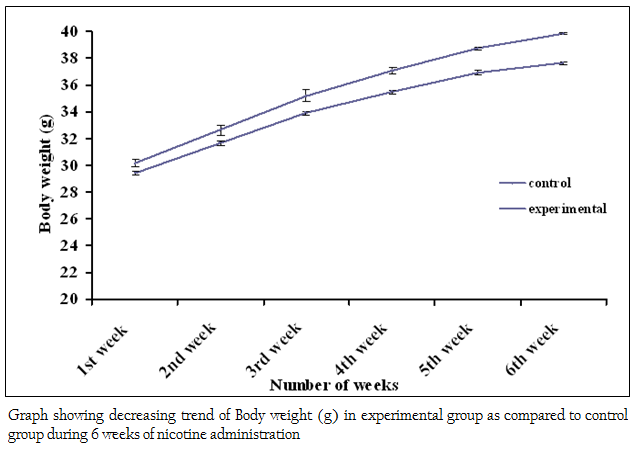
Figure 3: Graph showing decreasing trend of Body weight (g) in experimental group as compared to control group during 6 weeks of nicotine administration
RESULTS
Physical Performance
The body weights of all the adult male mice (n = 85) were measured on the first day of the acclimatizing period (1 month). The mean body weight was 19.95 ± 0.04 (g). The body weight of all adult male mice (n = 80) was recorded again at the end (30th day) of the acclimatizing period. The mean value was 26.51 ± 0.06 (g). During the whole acclimatizing period the gain in body weight was approximately 6.56 (g) (Figure 1). All the animals, which were used in the experiment, were very healthy and physically active. During the 6 weeks of experimental period it has been observed that the food intake as well as body weight of experimental mice was decreased as compared to the control group (Figure 2 and 3)
Hematological Parameters
The values of various hematological parameters including WBC, RBC, Hgb concentration, Hct, MCV, MCH, MCHC and PLT were assessed in control and experimental group (Table I). In experimental group t–test showed significant increase (p≤0.05) in WBCs count, PLT count, HCT and MCV in as compared to the control group. However, significant decrease (p≤0.05) in RBCs count, Hgb concentration, MCH and MCHC was observed in experimental group as compared to control group.
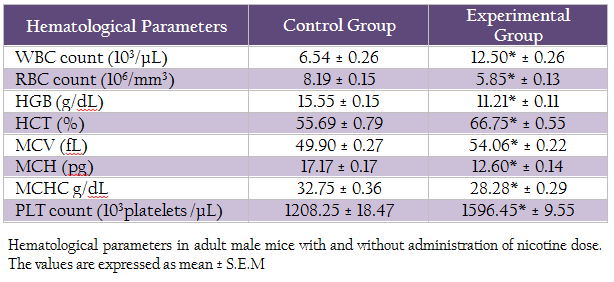
Table 2: Hematological parameters in adult male mice with and without administration of nicotine dose. The values are expressed as mean ± S.E.M
Lipid Profile
The mean ± SEM value of cholesterol level, triglycerides, HDL–C, LDL–C and HDL/LDL–C ratio was presented in Table II. The result of t– test statistic indicated the significant increase in cholesterol level, triglycerides level, LDL–C level in experimental group (p≤0.05) as compared to control group and significant decrease in HDL–C and HDL–C/LDL–C ratio.
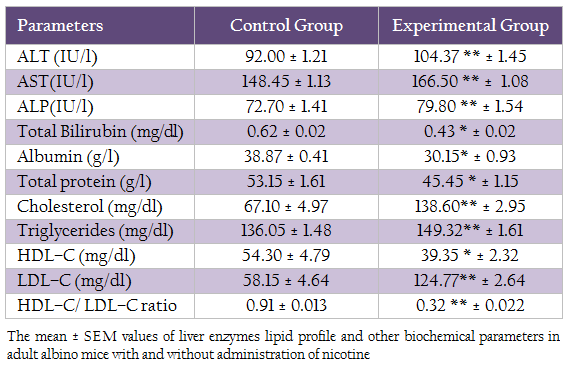
Table 2: The mean ± SEM values of liver enzymes lipid profile and other biochemical parameters in adult albino mice with and without administration of nicotine
Liver Enzymes
The mean values of liver enzymes (AST, ALT, ALP), total bilirubin, albumin and total protein was presented in table II. T–test statistic revealed that significant increase (p≤ 0.05) in ALT, AST, ALP after administration of nicotine in experimental group as compared to control group. Whereas, significant decrease (p ≤0.05) in total bilirubin, albumin and total protein was observed in experimental group as compared to control group.
DISCUSSION
The present study was undertaken to study the effects of nicotine on various hematological and biochemical parameters in mice. Our results showed a significant (p≤0.05) decrease in the body weight and food intake of nicotine–treated adult male mice as compared to control mice. The work of Audi et al., (2006) showed that administration of nicotine to rats caused a significant decrease in the body weight and food intake. The decrease in food intake and body weight caused by nicotine administration might be due to neuro–regulatory substances which effect food intake mechanism (Wack & Rodin 1982).
Herxheimer et al., (1967) reported that nicotine produces the same hemodynamic changes as cigarette smoking. Rausch et al., (1989) and Schwartz et al., (2005) reported that nicotine causes many changes in blood cells as it simply diffuses into the cells. This study confirmed that administration of nicotine to adult male mice significantly altered various hematological parameters including WBC, RBC, Hgb, HCT, MCV, MCH, MCHC and PLT.
A significant increase in WBCs count and decrease in RBCs count was observed. These results were similar to the results of Corre et al., (1971). The elevated WBCs count in our study is in agreement with the findings of other investigators including (Friedman et al., 1973; Okuno, 1973; Sutek & Jedrzejczak, 1973). One of the major effects of nicotine on the physiology of body is that it greatly suppresses the function of immune system and due to this reason the number of WBCs increased in the body to strengthen the immune system. (Geng et al., 1996)
It is documented that nicotine inhibits the function of erythrocytes, fibroblasts and macrophages. The work of Sherwin & Gastwirth (1990) and Siana et al., (1992) clearly showed that the administration of nicotine causes the diminished proliferation of red blood cells and as a result the RBCs count decreases. Low erythrocytes count may lead to a number of physiological disorders that may affect the efficiency of various enzymes.
In the present study it was observed that nicotine administration resulted in significantly (p≤0.05) decreased hemoglobin level. Similar results were also found by the work of Zafar et al., (2003). In our study the HCT level was found to be significantly (p≤0.05) higher in experimental animals as compared to control group. Ogston et al., (1970) showed that nicotine inhalation results in high level of HCT. One of the explanations for the apparent acute effect on the HCT level is that nicotine increases MCV also. MCV values were found to be significantly (p≤0.05) higher in our experimental group. Okuno (1973) demonstrated in his study that nicotine caused an increase in MCV. A significant decrease in both MCH and MCHC were also observed in this study.
The PLT count in present study was found to be significantly higher in experimental group. Literature reports on the effects of nicotine on PLT count seem to be controversial. De–Gactano et al., (1990) showed that nicotine caused platelet and leukocyte activation, and this resulted in the stimulation of platelet function.
Cholesterol level significantly increased in experimental group as compared to the control group and our findings were similar to the previous studies. Annida and Venugopal, (2007) described in their study that the level of free fatty acids, cholesterol and triglycerides increased in plasma of male albino Wistar rats treated with nicotine subcutaneously. The presences of hypercholesterolemia and triglyceridemia in heavy smokers are due to increased activity of 3–hydroxy–3–methyl–glutaryl CoA reductase and increased incorporation of labeled acetate into cholesterol. Chattopadhyay, (2008) also indicated that the administration of nicotine in adult albino rats caused a significant increase of total cholesterol and triglycerides.
There was significant increase in triglycerides (mg/dl) level in experimental group as compared to control group. Our results were according to the previous studies that reported increased cholesterol and triglyceride levels and decreased levels of HDL cholesterol (Gupta et al., 2004). Higher level of triglycerides occurred due to the presence of nicotine that decrease the activity of lipoprotein lipases and these enzymes involved in the uptake of circulating triglycerides rich lipoprotein and VLDL by the extra hepatic tissue (Cryer, 1981). It was observed that administration of nicotine decreases the total proteins, albumin and bilirubin but increased ALT, AST and ALP levels. Similar results were also reported by Jang et al., (2012).
CONCLUSIONS
It is concluded that intake of nicotine decreases the hematological parameters, decreases HDL with increase in TC, TG LDL–C and liver enzymes. These effects of nicotine on lipid profile and liver enzymes are responsible for a greater risk of developing atherosclerosis in the smokers leading to ischemic heart diseases.
ACKNOWLEDGEMENT
The authors acknowledge the financial support by HEC. (HEC funded project Ref No. PM/ IPFP /HRD /HEC /2010/ 1808).
CONFLICT OF INTEREST
There is no conflict of interest.
REFERENCES
Annida B, Venugopal PM (2007).Protective effect of hesperidin on nicotine induced toxicity in rats. Ind. J. Exp. Biol. 45: 194 – 202.
Audi SS, Abraham ME, Borker AS (2006). Effect of cigarette smoke on body weight, food intake and reproductive organs in adult albino rats. Ind. J. Exp. Biol. 44: 562 – 565.
PMid:16872045
Bain BJ, Rothwell M, Fether R, Brown J (1992). Acute changes in hematological parameters on cessation of smoking. J. Royal Soc. Med. 85: 80 – 81.
PMid:1538385 PMCid:PMC1294887
Benatar M (2007). Lost in translation: Treatment trials in the SOD1 mouse and in human ALS. Neurobiology of Disease 26(1): 1 – 13.
http://dx.doi.org/10.1016/j.nbd.2006.12.015
PMid:17300945
Chattopadhyay K, Chattopadhyay BD (2008). Effect of nicotine on lipid profile, peroxidation and antioxidants enzymes in females' rats with restricted dietary proteins. Ind. J. Med. Res. 127: 571 – 576.
PMid:18765876
Cooke JP, Bitterman H (2004). Nicotine and angiogenesis: a new paradigm for tobacco–related diseases. The Ann. Rev. Med. 36: 33 –40.
Corre F, Lellouch J, Schwartz D (1971). Smoking and leukocyte counts. Results of an epidemiologic study. Lancet. 2: 632 – 634.
http://dx.doi.org/10.1016/S0140-6736(71)80071-5
Cryer PE (1981). Diseases of the adrenal medulla and sympathetic nervous system. J. Endocrin. Meta. 25: 511 – 512.
De–Gaetano G, Evangelista V, Rajtar G, Del MA, Cerletti C. (1990). Activated polymorphonuclear leukocytes stimulate platelet function. Thrombosis Research Supplement. 11: 25–32.
http://dx.doi.org/10.1016/0049-3848(90)90388-S
Friedman GD, Siegelaub AB, Seltzer CC, Feldman R, Collen MF (1973). Smoking habits and the leukocyte count. Arch. Envir. Health. 26: 137 – 143.
http://dx.doi.org/10.1080/00039896.1973.10666241
PMid:4688852
Gawrylewski A (2007).The trouble with animal models. The Scientist. 21(7): 45 – 51.
Geng Y, Savage SM, Razan–Boroujerdi S, Sopori ML (1996).Effects of nicotine on the immune response. II Chronic nicotine treatment induces T cell energy. J. Immun. 156: 2384 – 2390.
PMid:8786295
Gupta R, Hitindergurm, John, Lomew (2004). Smokeless to¬bacco and cardiovascular risk. Arch. Intl. Med. 164: 17.
http://dx.doi.org/10.1001/archinte.164.17.1845
PMid:15451758
Herxheimer A, Griffiths RL, Hamilton B, Wakefield (1967).Circulatory effects of nicotine aerosol inhalations and cigarette smoking in man. Lancet. 2: 754 – 755.
http://dx.doi.org/10.1016/S0140-6736(67)91950-2
Jang ES, Jeong SH, Hwang SH, Kim HY, Ahn SY, Lee J, Lee SH, Park YS, Hwang JH, Kim JW, Kim N, Lee DH (2012). Effects of coffee, smoking, and alcohol on liver function tests: a comprehensive cross–sectional study. BMC Gastroenterology. 12: 145
http://dx.doi.org/10.1186/1471-230X-12-145
Neurath GB (1994). Aspects of the oxidative metabolism of nicotine. Clin. Invest. 72:190 – 195.
http://dx.doi.org/10.1007/BF00189309
PMid:8012160
Ogston D, Bennett NB, Ogston CM (1970). The influence of cigarette smoking on the plasma fibrinogen concentration. Atherosclerosis. 11: 349 – 352.
http://dx.doi.org/10.1016/0021-9150(70)90073-0
Okuno T. (1973).Smoking and blood changes. J. Med. Assoc., 225: 1387 – 1388.
http://dx.doi.org/10.1001/jama.225.11.1387
http://dx.doi.org/10.1001/jama.1973.03220390061022
Pausova Z, Paus T, Sedova L, Berube J (2003). Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: a case of gene environment interaction. Kidney Intl. 64: 829 – 835.
http://dx.doi.org/10.1046/j.1523-1755.2003.00172.x
PMid:12911532
Rausch JL, Fefferman M, Ladisich–Rogers DG, Menard M (1989). Effect of nicotine on human blood platelet serotonin uptake and efflux. Progress in Prog. Neuropsychopharmacol. Biol. Psychiatry. 13: 907 – 916.
http://dx.doi.org/10.1016/0278-5846(89)90042-0
Schwartz K, Weizman A, Rehavi M (2005). Decreased platelet vesicular monoamine transporter density in habitual smokers. European Neuro–psychopharmacology. 15: 235 – 238.
http://dx.doi.org/10.1016/j.euroneuro.2005.02.005
http://dx.doi.org/10.1016/j.euroneuro.2004.11.001
PMid:15695071
Sherwin MA, Gastwirth CM (1990).Detrimental effects of cigarette smoking on lower extremity wound healing. J. Foot Surg. 29: 84 – 87.
PMid:2181017
Siana JE, Frankild S, Gottrup F (1992).The effect of smoking on tissue function. J. Wound Care. 1 (2): 37 – 41.
Slotkin TA (2004). Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates.Toxicology Applied Pharmacology, 198: 132 – 151.
http://dx.doi.org/10.1016/j.taap.2003.06.001
PMid:15236950
Snyder MJ, Hsu E, Feyereisen R (1993).Induction of cytochrome P– 450 activities by nicotine in the tobacco horn worm, manduca sexta. J. Chem. Ecol. 19: 2903 – 2910.
http://dx.doi.org/10.1007/BF00980591
PMid:24248784
Sutek K, Jedrzejczak W (1973). The effect of cigarette smoking on the count and pattern of white blood cells. Wiad Lek J. 26: 725 – 728.
Valenca SS, Fonseca A, Hora K, Santos R, Porto LC (2004). Lung morphometry and MMP–12 expression in rats treated with intra peritoneal nicotine. Exp. Toxi. Path. 55: 393 – 400.
http://dx.doi.org/10.1078/0940-2993-00322
PMid:15088641
Wack JT, Rodin J (1982). Smoking and its effects on body weight and system of caloric regulation. Am. J. Clin. Nutr. 35: 36.
Zafar I, Khan NM, Nisar M, Rashida M, Shumaila B, Syed AM (2003). Effect of cigarette smoking on Erythrocytes, Leukocytes and Hemoglobin. J. Med. Sci. 3(3): 245 – 250.
http://dx.doi.org/10.3923/jms.2003.245.250




