Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (3): 159 – 163Experimentally Induced Liver Cirrhosis with Ascites by Carbon Tetrachloride and Phenobarbital Sodium in Wistar Rats
Saravanan Mani1*, Debratra Mondal2, Kalyan Sarma3, Karampal Singh4,
- Teaching Veterinary Clinical Complex, VCRI Orathanadu, Thanjavur – 614625
- Division of Medicine, Indian Veterinary research Institute, Izatnagr, Bareilly – 243 122 (UP) India
- Department of Veterinary Medicine, College of Veterinary Sciences & A.H. Central Agricultural University, Selesih, Aizawl, Mizoram
- Joint Directorate CADRAD, Indian Veterinary research Institute, Izatnagr, Bareilly – 243 122 (UP)
*Corresponding author: sara82vet@yahoo.com
ARTICLE CITATION:
Mani S, Mondal D, Sarma K, Singh K (2014). Experimentally induced liver cirrhosis with ascites by carbon tetrachloride and Phenobarbital sodium in Wistar rats. Adv. Anim. Vet. Sci. 2 (3): 159 – 163.
Received: 2014–01–12, Revised: 2014–01–31, Accepted: 2014–01–31
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.3.159.163
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
The study was conducted to evaluate liver cirrhosis with ascites by Carbon tetrachloride and Phenobarbital sodium in rat model. Phenobarbital sodium @ 0.3g/L in the drinking water for first week followed by Carbon tetrachloride (CCl4) along with olive oil (1:1 v/v) @ 2 ml per kg. B.wt was administered orally twice a week for 11 weeks. Reduction in Hb, PCV, TEC, TP, albumin, globulin and AG ratio while increased levels of TLC, total bilirubin, AST, ALT, and ALP were recorded. Estimation of oxidative stress indices revealed increased level of LPO along with decreased GSH, SOD and CAT. Liver showed severe fibrosis with cirrhotic nodule and 67% of rats were developed ascites. Carbon tetrachloride with Phenobarbital sodium can be used to induce hepatotoxicity (i.e. liver cirrhosis) with ascites in rat model.
INTRODUCTION
Liver plays vital role in human as well as animal body and it is the principal site for intense metabolism and excretion. It has a surprising role in the maintenance, performance and regulating homeostasis of the body. Liver involved with almost all the biochemical pathways to growth, fight against disease, nutrient supply, energy provision and reproduction (Ward and Daly, 1990). Ascites is the most common major complication of liver cirrhosis. Ascites is associated with poor quality of life, increased risk of infection, and renal failure (Runyon, 2004). The most validated cirrhosis model in the rat is produced with the use of the carbon tetrachloride (CCl4) along with Phenobarbital sodium (Proctor and Chatamra, 1984).
Carbon tetrachloride (CCl4) intoxication is a well–known model for producing oxidative stress and hepatic injury. Its biotransformation produces hepatotoxic metabolites, the highly reactive trichloromethyl free radical, which is further converted to the peroxytrichloromethyl radical (Williams and Burk, 1990). Reactive oxidant species may contribute to both onset and progression of fibrosis (Poli, 2000; Parola and Robino, 2001).
Many protocols exist, which differ in the route of administration (gavage, intra-peritoneal or subcutaneous injection), the dilution of CCl4 (1/20 to 1/10), the frequency (1 to 4 wk) and the duration (8 to 28 wk) of CCl4 administration. The efficiency of these protocols (70% to 100%), as well as the inherent toxic–mortality (20% to 90%) is variable in the literature (Mullen and McCullough, 1989). CCl4 protocol for cirrhosis is best described by various research workers (Doi et al., 1991; Abraldes et al., 2006). Hence, the study was intended to develop ascites of hepato-biliary disorder in rat model by CCl4 and Phenobarbital sodium.
MATERIALS AND METHODS
Experimental Model
Eighteen Wistar rats, weighting about 200–250g were used for this study. The rats were divided randomly into two groups namely healthy control (n=6) (Gr I) and disease control (Gr II) of 12 rats each. In the rats of group II, ascites was induced by Phenobarbital sodium @ 0.3g/L in the drinking water for first week (Abraldes et al., 2006) followed by carbon tetrachloride (CCl4) analar grade along with olive oil (1:1 v/v) @ 2 ml/ kg B.wt orally twice a week (Doi et al., 1991) for 11 weeks. The total experimental period was 12 weeks.
Clinical Signs and Body Weight
Experimental rats were observed closely for exhibition of any clinical and behavioral abnormality, and body weight was recorded at day 0, 2nd wk, 4th wk, 6th wk, 8th wk, 10th wk & 12th wks.
Samples Collection
Blood samples were collected at day 0, 4th, 8th & 12th wks for haemato-biochemical analysis to study the progressive changes in rat treated with CCl4. Plasma/ Serum samples were separated from blood collected with/without anticoagulant at 3000xg for 10 min for various biochemical parameters estimation.
Haematology Profile
Haemoglobin and haematocrit, total erythrocyte counts (TEC), total leukocytes counts (TLC) were estimated by standard protocols.
Serum Biochemical Profile
ALT, AST, ALP, Serum bilirubin, total protein, albumin, sodium and potassium were measured by using commercial kits (Span Diagnostic, Surat India).
Sacrificing
Liver was collected after sacrificing animals at the end of 12 week as per Committee for the Purpose of Control and Supervision on Experiments CPCSE) on animals norms for gross and histopathological examination and oxidative stress index was performed in liver tissue.
Estimation of Antioxidant Level
Assays of Oxidative Stress Markers in the Liver Tissue Samples
Estimation of various oxidative stress marker parameters in the liver tissue was executed. A double beam UV–VIS spectrophotometer (UV 5704 SS, ECIL, India) was used for recording the absorbance of the test samples.
Preparation of Liver Homogenates
For oxidative stress indices, 500 mg of liver tissue was taken in 5 ml of ice–cold PBS (pH 7.4). Another 200 mg liver tissue was taken in 2 ml of 0.02 M EDTA in distilled water and used for estimation of (reduced glutathione) GSH. The homogenate (10%) prepared with homogenizer under ice–cold conditions were centrifuged 3000xg for 10 min and finally supernatant was stored at –200C until assay.
Lipid Peroxidation (LPO) Assay
The concentration of Malonyldialdehyde (MDA), a reliable marker of lipid peroxidation, was estimated in tissue homogenate following the method suggested by Placer et al. (1966). Briefly, the reaction mixture consisted of 0.2 ml of 10% liver homogenate, 1.3 ml of 0.2 M Tris– 0.16 M KCl buffer (pH 7.4) and 1.5 ml of thiobarbituric acid reagent. The mixture was heated in boiling water bath for 10 min using glass beads as condenser. After cooling, 3ml of pyridine/n–butanol (15:1, v/v) and 1 ml of 1N sodium hydroxide (NaOH) were mixed by vigorous shaking. Optical density was measured spectrophotometrically at 532 nm against blank prepared by using distilled water. Lipid peroxidation was calculated on the basis of molar extinction coefficient of MDA–TBA complex at 548 nm i.e. 1.56 x 10 –5/mol/cm and expressed in terms of nmol of MDA/mg of protein.
Reduced Glutathione (GSH) Assay
The concentration of reduced glutathione in tissue homogenates was estimated by 5, 5–dithiobis–(2–nitro– benzoic acid) (DTNB) method as per the procedure of Sedlak and Lindsay (1968). Briefly, 1 ml of homogenate, 0.8 ml of water and 0.2 ml of 50% TCA solution were mixed and incubated at room temperature for 15 min. After incubation, the mixture was centrifuged at 3000xg for 15 min and 0.4 ml of supernatant was aspirated. Finally, 0.8 ml of 1M tris buffer and 0.2 ml of DTNB (0.01M) reagent was added and the optical density was measured spectro-photometrically at 412 nm within 5 min against blank prepared by using distilled water. Reduced glutathione concentration in the test sample was calculated by employing the molar extinction coefficient of DTNB–GSH conjugate (ηmol/mg Hb), 13600/M/cm. Moles DTNB–GSH conjugate formed per g wet tissue ( = [(OD/ε) X (total vol. of reaction/ volume of taken sample) X DF X 1000]. Where ε is the molar extinction coefficient of DTNB–GSH conjugate at 412 nm.
Superoxide Dismutase (SOD) Assay
SOD activity in liver homogenate was measured by using nitro blue tetrazolium as a substrate after suitable dilution as per the method of Menami and Yoshikawa (1979). The increase in absorbance due to auto oxidation of pyrogallol was measured at 420 nm. One unit of SOD activity was defined as the amount of enzyme that inhibited auto–oxidation by 50% under the given experimental condition and the values were expressed as U/mg of protein.
Catalase (CAT) Assay
Catalase activity in liver homogenate was estimated by using H2O2 as a substrate (Bergmayer, 1983). Time (seconds) required for the fall in the initial absorbance by 0.05 was recorded. One unit of activity is equal to mmol of H2O2 degraded per minute and is expressed as U/mg of protein.
Gross Pathology of Liver
Liver of all the rats were examined at the end of experiment for any gross pathological changes (Runnels et al., 1965).
Histopathological Study
The liver tissues were fixed in 10% formalin and embedded with paraffin. The sections were cut and stained with hematoxylin and eosin.
Statistical Analysis
All data are expressed as means ± SE unless otherwise specified. The analyses were performed with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The Student’s t–test and ANOVA were used to analyze the in–vitro experimental study.
RESULTS
Clinical Signs and Body Weight
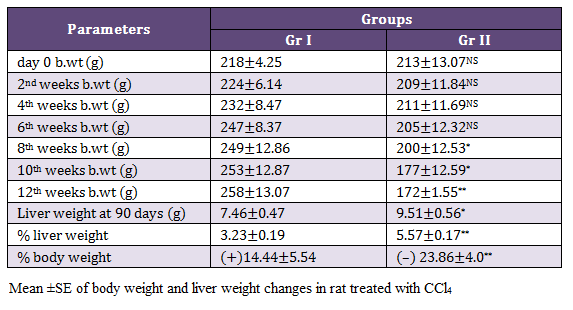
There were no mortality in rats exposed with CCl4 and 8 (67%) rats exhibited mild abdominal distension on 11th and 12th weeks of experiments. Gr II rats showed dullness and decrease feed intake from 11th weeks onwards. Significantly decreased (p < 0.05) body weight were noticed in Gr II from 8th, whereas highly significant reduction (p < 0.01) in the body weight was observed on 12th week as compared to rats of Gr I. There was significant increase (p < 0.05) liver weight at 90th day in the rats of Gr II as compared to healthy ones (Table 1).
Hematology Profile
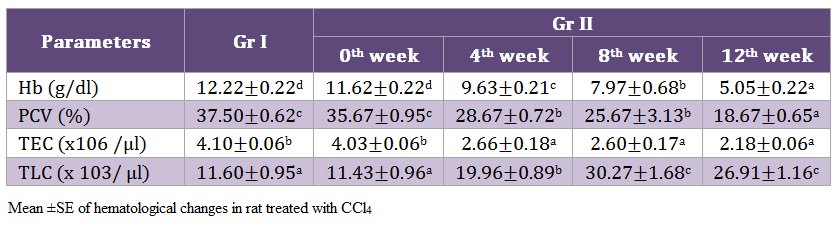
Highly significant (p < 0.01) decrease in hemoglobin was noticed on 12th week. Similarly, a significant (p < 0.05) reduction in PCV and TEC values while TLC values of Gr II rats increased by the end of experimentation (Table 2).
Serum Biochemical Profile
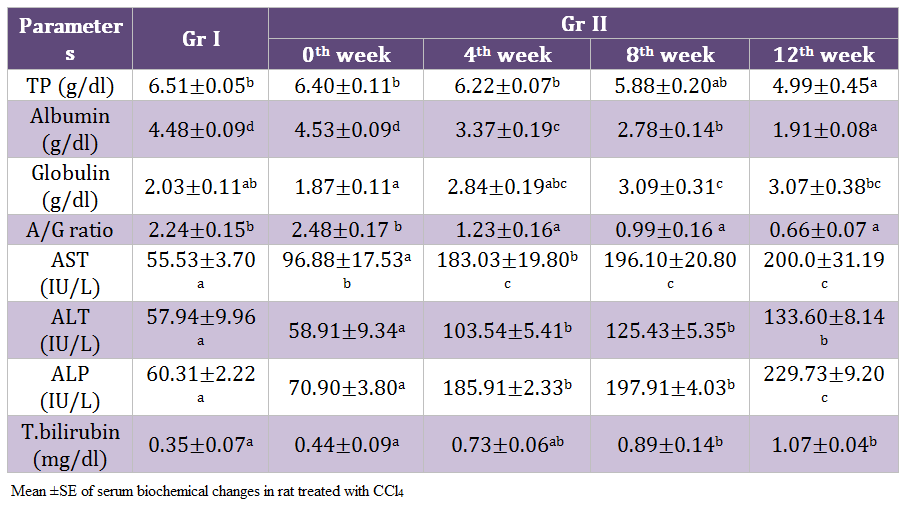
There was highly significant (p < 0.01) decrease in total protein, albumin and A:G ratio whereas significant increase (p < 0.05) in globulin, AST, ALT, ALP and total bilirubin was noticed on 12th weeks of experiment in the rats of Gr II as compared to healthy control. Gradual significant reduction in the albumin level of Gr II rats was observed during the experimentation. Values of A: G ratio in the rats of Gr II decreased significantly (p < 0.05). (Table 3)
Oxidative Stress Indices
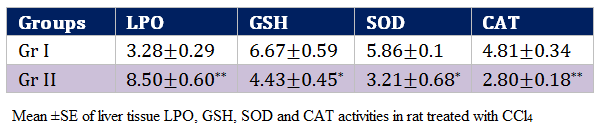
Oxidative stress indices of this study reflects highly significant (p < 0.01) increase in the values of LPO (8.50±0.60 nmol MDA/mg protein) and decrease in CAT values in the rats of Gr II as compared to Gr I by the end of experimentation. Values of GSH and SOD decreased significantly (p < 0.05) in the rats of Gr II as compared to Gr I (Table 4).
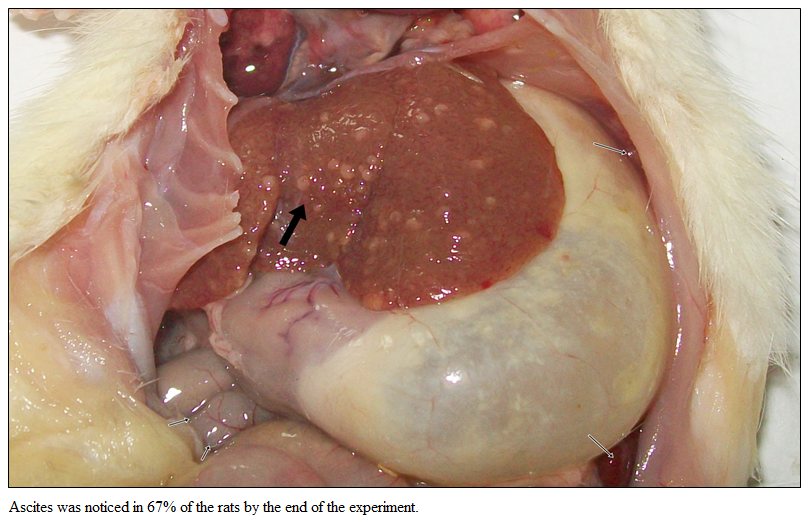
Gross Pathology of Liver
Gross examination of liver of Gr I showed normal liver without ascites. Rats of Gr II revealed severe fibrosis with cirrhotic nodule by the end of the experiment (Figure 1). Ascites was noticed in 67% of the rats by the end of the experiment.
Histopathological Study
Gr I (Healthy group) showed normal architecture of hepatic lobes and failed to reveal any specific pathological changes and was graded 0 by histopathological score system (HPS). Section of rat liver in Gr II revealed perilobular fibrosis and fatty changes which led to the separation of the lobules (Figure 2).
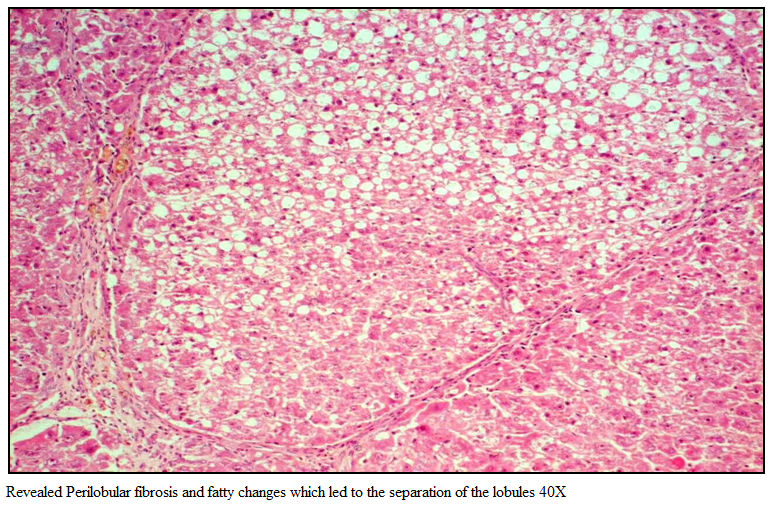
Figure 1: Revealed Perilobular fibrosis and fatty changes which led to the separation of the lobules 40X
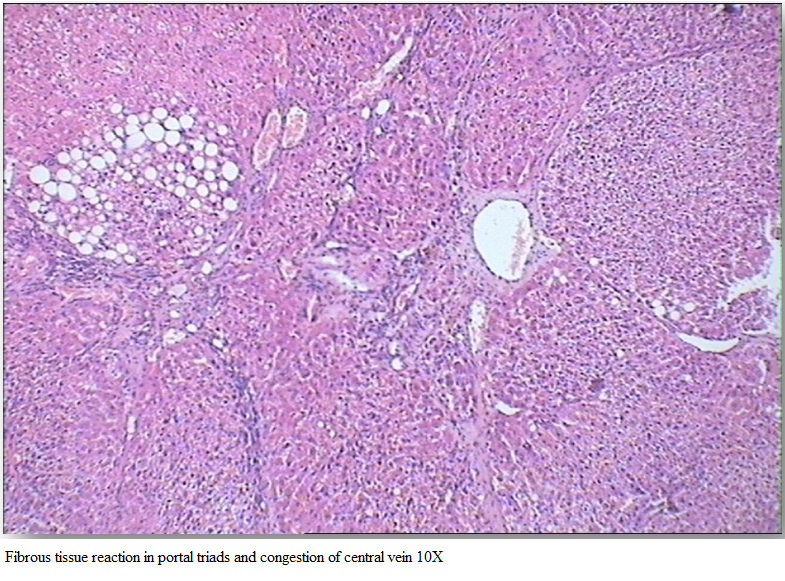
Hepatocytes showed cytoplasmic vacuolation in variable sizes, increased fibrous tissue reaction in portal triads and congestion of central vein (Figure 3). Liver tissue of Gr II was graded 3 by HPS system.
DISCUSSION
Induction of Ascites in Rat with CCl4 and Phenobarbital Sodium
The most validated cirrhosis model in the rat is produced using CCl4 along with phenobarbital (Proctor and Chatamra, 1984). CCl4 is one of the oldest and most widely used chemical agents for experimental induction of liver toxicity in laboratory animals (Domenicali et al., 2009). Chronic hepatotoxicity and cirrhosis in male sprague dawley rats could be produced by using CCl4 @ 2 ml +2 ml of olive oil per kg b.wt p.o twice a week for 12 wks (Doi et al., 1991). Continuous administration of CCl4 induces chronic liver injury that leads to cirrhosis. Phenobarbital (0.3 g/L) was added to drinking water to increase the yield of liver cirrhosis, starting one week before first CCl4 administration (Abraldes et al., 2006). Rats developed micro nodular cirrhosis, portal hypertension, portal systemic shunting and hyper dynamic changes in the circulation (Vorobioff et al., 1983) 12 –15 weeks after CCl4 administration. In the present study there were no mortality in rats exposed with CCl4 and 67% of rats exhibited mild abdominal distension on 11th and 12th weeks and rats showed ascites during autopsy. These findings were similar with Domenicali et al. (2005& 2009) who reported signs of hepatoxicity using oral phenobarbital for first week followed by CCl4 inhalation.
Clinical Signs and Body Weight
Nagano et al. (2007) recorded significant decrease in body weight and increase liver weight on administration of 18ppm CCl4. In the present study also, highly significant (p < 0.001) increase in percentage of liver weight and decrease in body weight were observed in CCl4 treated rats. The increase in liver weight could be attributed to the accumulation of fat (Reddy et al., 2010).
Haematology Profile
Significant reduction was recorded in hemoglobin concentration, PCV and TEC values while TLC values of Gr II rats exhibited an increase, indicating the presences of anaemia and leukocytosis. This anaemia and leukocytosis may be due to the direct effect of toxin on the haematopoietic system (Parent– Massin, 2004). Significant decrease in Hb and haematocrit values were noticed in male and female rats exposed to CCl4 @ 90 ppm (Nagano et al., 2007) possibly due to the toxic effect CCl4 on the hematopoietic system.
Serum Biochemical Profile
Hypoalbuminenia and hypoproteinemia are the useful index for determining the severity of hepatocellular damage (Aniya et al., 2005). Increased liver enzymes in CCl4 treated rats might be due to release of cytosolic enzyme into blood stream due to liver cell plasma membrane damage and loss of functional integrity of cell membrane in liver (Kazeem, et al., 2011). The increased levels of serum ALT and AST are indicative of cellular leakage and loss of functional integrity of cell membrane in the liver. Hepatocellular necrosis leads to elevation of the serum marker enzymes, released from the liver into blood like increased levels of serum ALT, AST, ALP and bilirubin are conventional indicators of liver injury which is supporting the present findings of Shenoy et al. (2002); Achliya et al. (2003).
Oxidative Stress Indices
In present investigation, significant (P < 0.01) elevation of lipid peroxidation in the liver of rats receiving CCl4 (Gr II) reflects membrane damage as alteration in its structure and function. The hepatotoxic effect of CCl4 is largely due to its active metabolite, trichloro methyl radical. The activated radicals bind covalently to the macromolecules and induce peroxidative degradation of membrane lipids of endoplasmic reticulum (ER) that is rich in polyunsaturated fatty acids (Hye, 2002). The increase in melondialdehyde levels in liver suggested enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms to prevent the formation of excessive free radicals (Shenoy et al., 2002). Significantly (P < 0.01) reduced activities of SOD, Catalase and GSH in group receiving CCl4 (Gr II) point out the hepatic damage (Kazeem et al., 2011). Regarding non–enzymatic antioxidants, GSH has been considered as a critical determinant of tissue susceptibility to oxidative damage and the depletion of hepatic GSH has been associated with an enhanced toxicity to chemicals, including CCl4 (Hewawasan et al., 2003). These reactive oxygen species can induce degenerative changes by oxidation of protein, peroxidation of lipids and damage to nucleic acids (Doelman and Bast, 1990). One of the principal causes of CCl4 induced liver injury is formation of lipid peroxides by free radical derivatives of CCl4 (CCl3). CCl4 induced adverse changes were evident from the decreased hepatic antioxidant enzyme ability viz., SOD, GPX followed by GSH of the present study. The body has an effective defence mechanism to prevent and neutralize the free radical–induced damage. This has been accomplished by a set of endogenous antioxidant enzymes such as SOD, catalase and GPX. These enzymes constitute a mutually supportive team of defense against ROS (Babe and Panemangalore, 2003). In CCl4 induced hepatotoxicity, the balance between reactive oxygen species (ROS) production and the antioxidant defense may be lost resulting in oxidative stress, which through a series of events deregulates the cellular functions leading to hepatic necrosis.
Gross Pathology of Liver
Gross examination of CCl4 treated (Gr II) rats liver revealed severe fibrosis with nodular cirrhosis with ascites in 67% rats by the end of experimentation. The results conformed to the observations of Vorobioff et al. (1983) who reported micro nodular cirrhosis, portal hypertension, portal systemic shunting and hyper dynamic changes in the circulation in 12–15 wks after CCl4 administration. Domenicali et al. (2005 and 2009) also reported the development of ascites between 11th and 15th weeks with oral phenobarbital for first week and followed by CCl4 inhalation in rat.
Histopathological Study
Hepatic damage produced by CCl4 was characterized by fatty changes, centrilobular necrosis, hydropic degeneration and cirrhosis. Doi et al. (1991) and Huang et al. (2006) also reported similar changes following CCl4 administration in rats.
CONCLUSSION
Oral administration of Phenobarbital sodium followed by Carbon tetrachloride (CCl4) could be used for induction of liver cirrhosis with ascites in wistar rats.
ACKNOWLEDGEMENTS
Authors are extremely grateful to the Director, Indian Veterinary Research Institute, Izatnagar for providing all facilities for the study
REFERENCES
Abraldes JG, Pasarin M, Garcia–Pagan JC (2006). Animal model of Portal hyper–tension. World J. Gastroenterol. 12 (41): 6577 – 6584.
PMid:17075968
Aniya Y, Koyama T, Miyagi C, Miyahira M, Inomata C. Kinoshita S, Ichiba T (2005). Free radical scavenging activity and hepatoprotective actions of medicinal herb, Crassocephalum crepidioides from the Okinawa islands. Biol. Pharm. Bull. 28: 19 – 23.
http://dx.doi.org/10.1248/bpb.28.19
PMid:15635156
Babe FN, Panemangalore M (2003). Exposer to low doses of endosufan and chlor–pyriphos modifies endogenous antioxidants in tissues of rats. J. Environ. Sci. Health. 38:349–363.
http://dx.doi.org/10.1081/PFC-120019901
PMid:12716052
Bergmayer HU (1983). UV method of catalase assay. In: Methods of Enzymatic Analysis, Deer Field Beach, Florida, 3: 273.
Doelman CJA, Bast A (1990). Oxygen radicals in lung pathology. Biol. Med. 9: 381 – 400.
Doi K, Kurab S, Shimazu N, Inagaki M (1991). Systemic histopathology of rats with CCl4– induced hepatic cirrhosis. Lab. Anim. 25: 21 – 25.
http://dx.doi.org/10.1258/002367791780808121
PMid:2010972
Domenicali M, Caraceni P, Giannone F, Baldassarre M, Lucchetti G, Quarta C, Patti C, Catani L, Nanni C, Lemoli RM, Bernardi M (2009). A novel model of CCl4–induced cirrhosis with ascites in the mouse. J. Hepatol. 51: 991 – 999.
http://dx.doi.org/10.1016/j.jhep.2009.09.008
PMid:19853952
Domenicali M, Caraceni P, Principe A, Pertosa AM, Ros J, Chieco P, Trevisani F, Jimenez M, Bernardi M (2005). A novel sodium overload test predicting ascites decompensation in rats with CCl4–induced cirrhosis. J. Hepatol. 43: 92 – 97.
http://dx.doi.org/10.1016/j.jhep.2005.01.034
PMid:15893844
Hewawasan RP, Jayatilaka KAPW, Pathirana C, Mudduwa LKB (2003). Protective effect of Asteracanttha longifolia extracts mouse liver injury induced by carbon tetrachloride and paracetamol. J. Pharmacol. 55: 1413 – 1418.
http://dx.doi.org/10.1211/0022357021792
PMid:14607024
Huang YH, Mei–Na Shi, Wei–Da Zheng, Li–Juan Zhang, Zhi–Xin Chen, Xiao–Zhong Wang (2006). Therapeutic effect of interleukin–10 on CCl4–induced hepatic fi brosis in rats. World J. Gastroenterol. 12(9): 1386 – 1391.
PMid:16552806
Hye GJ (2002). Hepatoprotective effect of 18b– Glycyrrhetinic acid on carbon tetrachlorideinduced liver injury: inhibition of cytochrome P4502E1 expression. Pharmacol. Res. 46: 221 –227.
http://dx.doi.org/10.1016/S1043-6618(02)00121-4
Kazeem MI, Bankole HA, Fatai AA (2011). Protective effect of ginger in normal and carbon–tetrachloride– induced hepatotoxic rats. Anal. biol. Res. 2 (1): 1 – 8.
Menami M, Yoshikawa H (1979). Simplified assay method of superoxide dismutase activity of clinical use. Clin. Chem. Acta. 92: 337 – 342.
http://dx.doi.org/10.1016/0009-8981(79)90211-0
Mullen KD, McCullough AJ (1989). Problems with animal models of chronic liver disease: suggestions for improvement in standardization. Hepatol. 9: 500 – 503.
http://dx.doi.org/10.1002/hep.1840090326
Nagano K, Umeda Y, Saito M, Nishizawa T, Ikawa N, Arito H, Yamamoto S, Fukushima, S (2007). Thirteen–week inhalation toxicity of carbon tetrachloride in rats and mice. J. Occup. Health. 49(4): 249 – 259.
http://dx.doi.org/10.1539/joh.49.249
PMid:17690517
Parent– Massin D (2004). Haematotoxicity of trichothecenes. Toxicol. Lett. 153 (1): 75 – 81.
http://dx.doi.org/10.1016/j.toxlet.2004.04.024
PMid:15342083
Parola M, Robino G (2001). Oxidative stress–related molecules and liver fibrosis. J. Hepatol. 35: 297 – 306.
http://dx.doi.org/10.1016/S0168-8278(01)00142-8
Placer ZA, Cushman LL, Johnson BT (1966). Estimation of product of lipid per–oxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 16: 359 – 364.
http://dx.doi.org/10.1016/0003-2697(66)90167-9
Poli G (2000). Pathogenesis of liver fibrosis: role of oxidative stress. Mol. Aspects Med. 21: 49 – 98.
http://dx.doi.org/10.1016/S0098-2997(00)00004-2
Proctor E, Chatamra K (1984). Standardized micronodular cirrhosis in the rat. Eur. Surg. Res. 16: 182 – 186.
http://dx.doi.org/10.1159/000128407
PMid:6723720
Reddy J, Ganasekaran D, Vijay D, Ranganathan TV (2010). Studies on hepatopro–tective activity of traditional ayurvedic formulation VIDAKANA CHOORNAM against CCL4 induced hepatotoxicity in albino rat. Int. J. Pharm Analy. 2(2): 5 – 16.
Runnels RA, Monlux WS, Monlux AW (1965). Principles of Veterinary Pathology. 7th edn., Scientific Book Agency, Calcutta.
Runyon BA (2004). Management of adult patients with ascites due to cirrhosis. Hepatol. 39: 841 – 854.
http://dx.doi.org/10.1002/hep.20066
PMid:14999706
Sedlak J, Lindsay RH (1968). Estimation of total protein bound and non protein sul–phydryl groups in tissues with Elman,s reagent. Anal. Biochem. 25: 192 – 205.
http://dx.doi.org/10.1016/0003-2697(68)90092-4
Shenoy KA, Somayaji SN, Bairy KL (2002). Evaluation of hepatoprotective activity of Gingo biloba in rats. Indian J. Pharmacool. 46(2): 167 – 174.
Vorobioff J, Bredfeldt JE, Groszmann RJ (1983). Hyper dynamic circulation in portalhypertensive rat model: a primary factor for maintenance of chronic portal hyper–tension. Am. J. Physiol. 244: G52 – G57.
PMid:6849394
Ward FM, Daly MJ (1999). Hepatic Disease. In: Clinical Pharmacy and Therapeutics, Walker R and Edwards C (Ed), Churchill Livingstone, New York, 195 – 212.
Williams T, Burk RF (1990). Carbon tetrachloride hepatotoxicity: an example of free radical–mediated injury. Semin. Liver Dis. 10, 279 – 284.
http://dx.doi.org/10.1055/s-2008-1040483
PMid:2281335