Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (3): 150 – 154Comparative Efficacy of Different Cryoprotectants for Deep Freezing of Buffalo Bull Semen
Ijaz Ahmad1, Sana Ullah Jatoi1, Muhammad Zubair1*, Muhammad Younis2
- Department of Theriogenology, Faculty of Veterinary Sciences, University of Agriculture Faisalabad, Pakistan
- Semen Production Unit Quadrabad, Department of Livestock Punjab, Pakistan
*Corresponding author: drzubairabbasi@gmail.com
ARTICLE CITATION:
Ahmad I, Jatoi SU, Zubair M, Younis M (2014). Comparative efficacy of different cryo-protectants for deep freezing of buffalo bull semen. Adv. Anim. Vet. Sci. 2 (3): 150 – 154.
Received: 2014–01–09, Revised: 2014–02–04, Accepted: 2014–02–05
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.3.150.154
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Different cryo-protectants are commonly used to reduce the chances of sperm damage during freezing of semen. Comparative effects of three glycerol extenders A, B and C with 7% glycerol, anhydrous glycerol 5% and 7% respectively were studied on sperm motility, livability and livability index during the post freezing technique of buffalo bull semen at Semen Production Unit Quadrabad. The six Nilli–Ravi Buffalo bulls were divided into two groups on the basis of age, three into each group. In group I animals were selected from 3 to 5 years of age and in group II above from 5 years of age. Motility percentages after thawing in three extenders A, B and C were 46.88 ± 7.27, 54.69±7.85 and 61.24± 9.64 for three extenders respectively for group I. For group II the motility percentage after thawing in three extenders A, B and C were 48.13± 5.27, 57.19±7.85 and 64.38± 9.64 for three extenders respectively. The Livability after thawing for three extenders A, B and C were 4.56±1.20, 5.17±1.20 and 6.61±1.16 hrs respectively for group I. For group II values livability for three extenders A, B and C were 4.39±2.20, 5.33± 1.33 and 6.44±1.29 hrs respectively. Absolute index of livability for three extenders A, B and C averaged 136.83±35.27, 149.06±36.18 and 170.61±32.16 respectively for group I. For group II values of absolute index of livability for three extenders A, B and C were 125.61±25.27, 128.06±19.64 and 146.83±24.46 respectively. It was concluded that the extender C (containing 7 % anhydrous glycerol) showed best results in terms of motility after thawing and livability at 37°C followed by extender B ( containing 5 % anhydrous glycerol) and extender A (containing 7% glycerol).
INTRODUCTION
genetic improvement of livestock. In this technique, the best quality semen collected from genetically superior sire, is evaluated, diluted and stored in the liquid or frozen form. The semen which is stored in liquid form after cooling to 5°C can be stored for 24–48 hours without the reduction in its motility. Through the cooling method, the semen which is stored for short time, many adverse effects are manifested in the form of reduction of structural integrity, motility of sperms and conception rates (Medeiros et al., 2002). For the storage of semen for indefinite period, the liquid nitrogen with temperature of –196°C is preferred as it yields adequate fertilization rates (Hammerstedt et al., 1990). However, cryopreservation is detrimental to spermatozoa and 50% of them lose their fertilizing capacity (Lessard et al., 2000). The process of cryopreservation decreases the sperm fertility through structural changes in plasma membrane, reduction of mitochondrial functions and reduction of motility (Chaveiro et al. 2006). There are many factors which influence the success rate of cryopreservation including compositions of extender, cooling rate, packing and thawing rate and variations within species (Clulow et al., 2008). To overcome these problems many cryoprotectants are used but glycerol has unique characters like penetrating within spermatozoa (Amann et al., 1993). Mostly 6 to 7 % concentrations of glycerol in extender are used commonly for freezing of buffalo bull semen but attempts are made to reduce the concentration of glycerol to 2–3 % and above 7% (Nastri et al., 1994). To get the maximum advantage from artificial insemination, it is necessary to halt the metabolic activities of spermatozoa for storage of longer time. The discovery of glycerol has made it possible due to its unique cryprotective activities. Various cryoprotective agents have been used but glycerol is the most widely used cryoprotectant for bull spermatozoa.
However, there is little information regarding the usefulness of anhydrous glycerol in deep freezing of buffalo bull semen. Therefore the present study was planned.
To compare the cryoprotective properties of hydrous and hydrous glycerol for deep freezing of buffalo bull semen collected from buffalo bulls of two age groups to study the post freezing motility, Livability and livability index of spermatozoa
MATERIALS AND METHODS
Experimental Animals
Six adult Nilli–Ravi buffalo bulls which were clinically without any reproductive problem were selected for the present study at semen Production Unit (SPU), Quadrabad, District Sahiwal, Pakistan. This region is situated at an altitude of about 173m and lies between longitudes 73° and 74°E and latitudes 30° and 30.15°N 9 (Ahmad et al., 1981).The age of bulls vary from 3 to 9 years. The bulls were divided into two groups; group I, 3 to 5 years of age Group II, above 5 years of age. The bulls were maintained under the natural climatic conditions of that region. To save the animals from heat stress, two measures were adopted; one was location of sheds with North–South for cross ventilation and second was plenty of water for wallowing. The seasonal green fodder was provided to animals with 10% body weight of bulls. Clean water was provided for 24 hours. Vaccination against Hemorrhagic Septicemia (HS) and Foot & Mouth Disease (FMD) was done as per schedule. Preventive measures against worm infestation were also undertaken.
Semen Collection and Initial Evaluation
The collection of semen was performed with artificial vagina. The sexual stimulation of bulls was done by allowing the 1–2 false mounts (Younis et al., 1998). At the time of each collection, two ejaculates were collected from each bull. The collected semen was evaluated immediately for mass activity, motility, morphology and sperm count. In this way, a total of 24 pooled semen samples, with eight samples for each bull, were used for further processing.
Extension and Freezing of Semen
The pooled semen samples from all bulls were diluted at 37°C by slow and one step method dilution method, using the three extenders A, B and C (Table1). Each semen sample was extended according to its sperm density to achieve a final concentration of 30×106 spermatozoa per insemination dose (0.5ml). After dilution, the motility percentage was recorded before filling of straws.
After recording post extension sperm motility, samples were cooled to 22°C, filled in 0.5 ml French straws and equilibrated for 4 hours at 4°C. Then samples were frozen in the liquid nitrogen first by holding the straws in vapors 5 cm above the surface of the liquid nitrogen for 8 minutes (Fiaz et al., 2010). Then straws were dipped in the liquid nitrogen for storage
Evaluation of Frozen Semen
After 24 hrs of storage in liquid nitrogen, the straws were thawed for 30 seconds in a water bath at 37°C and evaluated for sperm motility, livability and absolute index of livability and morphology. In order to determine the livability of spermatozoa, the thawed semen samples were kept at 37°C and the motility was observed at hourly intervals, till the death of the last sperm. Absolute index of livability of spermatozoa for each sample was computed as described by (Younis et al., 1998).
Data Analysis
Mean (+SE) values of various parameters for bulls of two age groups and three extenders were analyzed through 2×3 factorial experiment through ANOVA using completely randomized design. Duncan’s multiple range tests was applied for comparisons of means (Steel and Torrie, 1982)
RESULTS
Motility Percentage after Extension
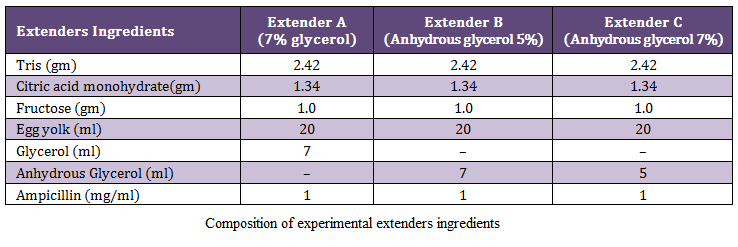
The motility percentage of age group I in extender A ranged from 60 to 75 percent with a mean of 62.71+3.61 percent while in extender B, it ranged from 60 to 80 percent with a mean 66.67±6.20 percent and in extender C it ranged from 60 to 90 percent with a mean of 73.54+8.40 percent. The motility for group II in extender A ranged from 60 to 75 percent with a mean of 64.79 + 5.41 percent while in extender B it ranged from 60 to 85 percent with a mean of 7 1.88 ± 7.34 percent and in extender C it ranged from 65 to 90 percent with a mean of 80.00 ± 8.60 percent as represented in (figure1) which represent significant difference among the extenders (P < 0.0l). They ranked as C, B and A on the basis of motility percentage after extension. The highest motility was extender C, while the lowest in extender A.
Post thaw Motility Percentage
The value of post thaw motility percentage for group I in extender A ranged from 30 to 60 with mean of 46.88 ± 7.27 while in extender B it ranged from 40 to 75 with mean of 54.69±7.85 and in extender C it ranged from 40 to 80 with mean of 61.25±I0.57.
In group 2 the values of post thaw motility percentage in the extender A ranged from 40 to 55 with mean of 48.13 + 5.74 while in extender B it ranged from 40 to 70 with mean of 57.19 + 7.30 and in extender C it ranged from 50 to 80 with mean of 64.38 + 9.64. Comparison of means are presented in figure (II) which represent significant difference among the extenders (P < 0.01). They fell in the C, B and A order on the basis of post thaw motility percentage.
Livability (hours) of Post Thaw Spermatozoa
The livability of spermatozoa of group 1 ranged from 3 to 7 hours with mean of 4.56±l.20 hours for extender A while in extender B it ranged from 3 to 7 hours with mean of 5.171±.20 hours and in extender C it ranged from 4 to 8 hours with mean of 6.6 1 ±1.14 hours. Livability (hours) for group II in extender A ranged from 3 to 6 hours with mean of 4.39 + 2.20 hours while in extender B it ranged from 3 to 7 hours with mean of 5.33+1.33 hours and in extender C it ranged from 4 to 9 hours with mean of 6.44±l.29 hours. Comparison of means represented in figure 3 represents significant difference (P < 0.01) among the extenders.
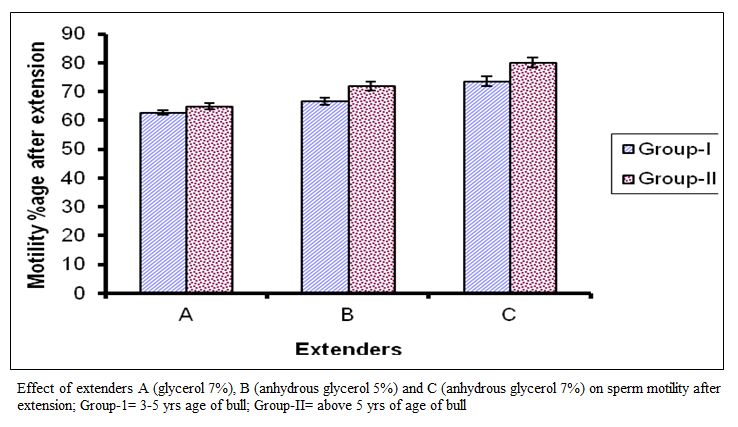
Figure 1:Effect of extenders A (glycerol 7%), B (anhydrous glycerol 5%) and C (anhydrous glycerol 7%) on sperm motility after extension; Group-1= 3-5 yrs age of bull; Group-II= above 5 yrs of age of bull
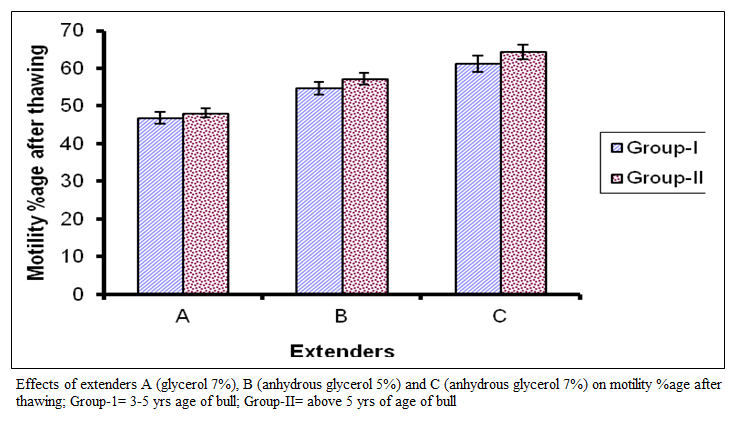
Figure 2:Effects of extenders A (glycerol 7%), B (anhydrous glycerol 5%) and C (anhydrous glycerol 7%) on motility %age after thawing; Group-1= 3-5 yrs age of bull; Group-II= above 5 yrs of age of bull
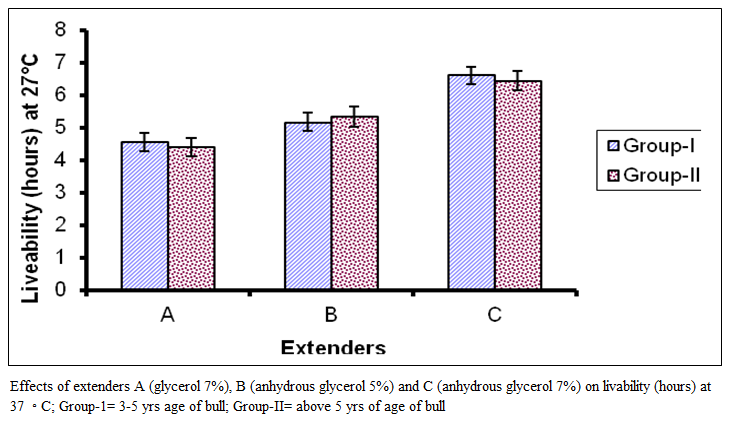
Figure 3:Effects of extenders A (glycerol 7%), B (anhydrous glycerol 5%) and C (anhydrous glycerol 7%) on livability (hours) at 37 ◦C; Group-1= 3-5 yrs age of bull; Group-II= above 5 yrs of age of bull
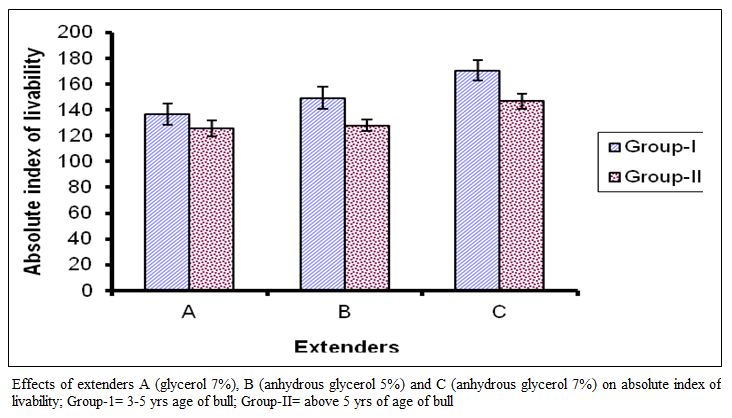
Figure 4:Effects of extenders A (glycerol 7%), B (anhydrous glycerol 5%) and C (anhydrous glycerol 7%) on absolute index of livability; Group-1= 3-5 yrs age of bull; Group-II= above 5 yrs of age of bull
The best results were observed in extender C, followed by extender B and A. However, the effect of bulls within a group on livability (hours) was non– significant.
Absolute Index of Livability
The absolute index of livability (Figure 4) for group I in extender A ranged from 80 to 185 with a mean of 136.85 + 35.27 while in extender B it ranged from 85 to 215 with a mean 149.06 + 36.18 and in extender C it ranged from 120 to 220 with a mean of I70.6 I +32.16.
For group II the absolute index of livability in extender A ranged from 85 to 182 with a mean of I 25.6 I ±26.63, while in extender B it ranged from 100 to 180 with a mean of 128.06+19.64 and in extender C it ranged from 114 to 200 with a mean of 146.83 + 24.46. In figure 4 comparison of means are presented which show significant difference among the extenders (P < 0.01). On basis of absolute index of livability the extenders ranked as C, B and A. The effect of age group was significant (P < 0.0I), the value was lower in bulls of group I than group in II.
DISCUSSION
Based on the finding of the present study, the extender C containing 7 percent anhydrous glycerol gave better results in term of motility percentage after extension, motility percentage after thawing, livability (hours) and absolute index of livability in freeze thawed buffalo bull spermatozoa, followed by extender B containing 5 percent an– hydrous glycerol and extender A containing 7 percent glycerol. The effect of bulls within a group on motility percentage after extension, post–thaw motility was non–significant. However, the values of absolute index of livability were lower in younger bull of group I than group in II.
There was a significant difference among extenders and extender C differed significantly in both the age groups after extension of semen. These observations were in line with (Qureshi et al., 1988) reported motility percentage after extension averaged 55.4I and 60.63 for two bulls. Our results were also in close agreement to (Swelum et al., 2011) and (Rohilla et al., 2005) also reported the sperm motility 72.14± 1.47 and 71.10 ±0.41 in semen of buffalo bull respectively.
The results of post thaw sperm motility also revealed that in extender C containing 7 percent anhydrous glycerol gave better results in term of post thaw motility percentage which is comparable to that found by (Chinnaiya et al., 1979), who found better results with the use of 7 percent anhydrous glycerol. Similar findings were reported by (Abbas and Andrabi, 2002) who reported 56 ±60 percent motility with 7 percent glycerol during the post–thaw motility.
Livability of spermatozoa after thawing at 37°C was significant in extender C as compared to B and A in both groups. Sadiq (1989) reported average livability of 9.8 ± 1.2 and 9.4 ± 0.7 hours for buffalo bull. The similar kinds of results were reported by (Younis et al., 1998) where overall mean Livability of spermatozoa was 5.04±0.11 hours. Siddique et al., (2006) reported the similar kind of findings with 7% glycerol of livability at 37oC 8.6 ± 0.9, 7.6 ± 0.9, 9.2 ± 1.1, 8.3 ± 0.9 and 7.6 ± 1.0 hours. These result showed the significant difference among extenders A, B and C. Extender C differed significantly with that of extender A and B.
There was significant difference among age groups in absolute index of Livability. The Livability index of frozen thawed semen for group 1 and group II in extenders A, B and C were in line with that of (Younis et al., 1998) who reported Livability index of spermatozoa I52.84± 1 3.68.
Among the three extenders used in the present study, the extender C (containing 7% anhydrous glycerol) differed significantly from extender B (containing 5% anhydrous glycerol) and extender A (containing 7% glycerol). Extenders B and A also differed significant (P < 0.05) in terms motility percentage after extension, motility percentage after thawing, livability of spermatozoa at 37°C and absolute index of livability. This difference may be due to higher binding ability of anhydrous glycerol which resulted in a change in the shape of ice crystals formed during freezing (Rapatz, 1966). The glycerol has the ability to make the hydrogen bonding with water, permeable with cell membrane and has no effect of toxicity between the concentration of 1–5mol/l (Fuller and Paynter 2004)
More particularly, the glycerol replaces the intracellular water during the process of cryopreservation and decreases the freezing point of water and resultant reducing the concentration of electrolytes in the unfrozen part so that low ice crystals are formed at any temperature (Holt 2000; Medeiros et al. 2002). It changes the size and shape of ice crystals during freezing process (Smith et al., 1951; Sherman 1957; Rapatz, 1966) and hence it reduces the mechanical destructiveness to sperm cell. Lovelock (1953) reported that glycerol acts through "a salt buffering" mechanism. It binds water and markedly decreases the freezing point of solution and less ice is formed in its presence at any given temperature (Stein, 1962). Lohman et al., (1964) stated that glycerol forms complex with metallic ions and reduces the chances of injury to sperms.
Anhydrous glycerol (due to purity 99%) is also oxidized by sperm cell, penetrates the cell rapidly and may reduce the mechanical destructiveness to sperm cell to a higher extent than hydrous glycerol. Salt buffering mechanism of glycerol may be enhanced by the use of anhydrous glycerol. It may also bind water and markedly decrease the freezing point of solution and less ice is formed due to its presence at any given temperature. Motility percentage after extension, motility percentage after thawing, livability and absolute index of livability were improved in anhydrous glycerol than glycerol due to its purity.
CONCLUSION
It was concluded from this experiment that pure glycerol produces the good results in freezing of semen of buffalo bull. Motility of buffalo bull is improved during cryopreservation, so pure glycerol should be used for freezing.
REFERENCES
Abbas A, Andrabi SMH (2002). Effect of different glycerol concentrations on motility before and after freezing, recovery rate, longevity and plasma membrane integrity of Nili–Ravi buffalo bull spermatozoa. Pak. Vet. J. 22: 1 – 4.
Ahmad N, Chaudhry RA, Khan BB (1981). Effect of month and season of calving on the length of subsequent calving interval in Nili–Ravi buffaloes. Anim. Reprod. Sci. 3: 301 – 306.
http://dx.doi.org/10.1016/0378-4320(81)90005-1
Amann RP, Graham JK (1993). Spermatozoal function. In: DiRienzi D, ed. Equine reproduction. Philadelphia: Lea and Febiger; 715 – 745.
Chinnaiya GP, Sharma PA, Ganguli NC (1979). Fertility study on frozen buffalo Semen. Ind. J. Dairy. Sci. 32: 93 – 94.
Clulow JR, Mansfield L J, Morris LHA, Evans G, Maxwell WMC (2008). A comparison between freezing methods for the cryopreservation of stallion spermatozoa. Anim. Reprod. Sci. 108: 298 – 308.
http://dx.doi.org/10.1016/j.anireprosci.2007.08.014
PMid:18065170
Fiaz M, Usmani R H, Abdullah M, Ahmad T (2010). Evaluation of semen quality of Holstein Friesian and Jersey bulls maintained under subtropical environment. Pak. Vet. J. 30: 75 – 78.
Fuller B, Paynter S (2004) Fundamentals of cryobiology in reproductive medicine. Reprod. Biomed. Online. 9: 680 – 691.
http://dx.doi.org/10.1016/S1472-6483(10)61780-4
Hammerstedt RH, Graham JK, Nolan JP (1990). Cryopreservation of mammalian sperm: what we ask them to survive. J. Androl. 11: 73 – 88.
PMid:2179184
Holt WV (2000). Basic aspects of frozen storage of semen. Anim. Reprod. Sci. 62: 3 – 22.
http://dx.doi.org/10.1016/S0378-4320(00)00152-4
Lessard C, Parent S, Leclerc P, Bailey JL, Sullivan R (2000). Cryopreservation Alters the Levels of the Bull Sperm Surface Protein P25b. J. Androl. 21: 700 – 707.
PMid:10975417
Lovelock JE (1953). The mechanism of protective action of glycerol against hemolysis by freezing and thawing. Biochem. Biophys. Acta. 11: 28 – 36.
http://dx.doi.org/10.1016/0006-3002(53)90005-5
Medeiros C, Forell MOF, Oliveria ATD, Rodrigues JL (2002). Current status of sperm cryopreservation: why isn't it better? Theriogenol. 57: 327 – 44.
http://dx.doi.org/10.1016/S0093-691X(01)00674-4
Nastri MJF, Del-Sorbo C, Fabbrocini A, Fasano G, Sansone G (1994). Performances motility in cooled and freeze thawed B. bubalis spermatozoa at different osmotic pressures. In: Bradley
Qureshi ZI, Ahmad N, Samad HA, Ala–ud–Din, Nawaz M (1988). Studies on effect of age diluted semen and double insemination on conception rate in buffalo. Pakistan. Vet. J. 8: 9 – 13.
Rapatz GL (1966). What happens when semen is frozen? Proc. First. NAAB Tech. Conf. Artif. Isum. Bovine Reprod. pp.45 – 56.
Rohilla RK, Tuli RK, Goyal RL (2005). Comparative study of the effects of cryoprotective agents in freezing Murrah buffalo bull semen. Indian. J. Vet. Res. 14: 37 – 43.
Sadiq AM (1989). Effect of tris based extenders modified with saccrides on freezibility of buffalo bull semen. M.Sc. thesis. Deptt. Anim. Reprod. Univ. Agri. Faisalabad.
Sherman A, Sail KH, Singh DD, Eliwadi, Fluekigar A (1957). Deep freezing of buffalo semen using egg yolk for citrate and citric acid whey extenders. Its performance in the field. Indian Vet. J. 56: 1017 – 1019.
Siddique M, Ali R, Raza A (2006). Effect of Buffers on Freezing of Buffalo Bull Semen. J. Agri. Social Sci. 2: 117 – 119.
Smith A, Polage UC, Smiles V (1951). Microscopic observation of liviving cells during freezing and thawing.4th FAO Swedidsh Int. Post Graduate course in Anim.Reprod.Swedon. J. Royal Microscopic Society. 71: 186 (Anim. Breed. Abst. 21(2): 976: 1953.
Steel RGD, Torrie JH (1982). Principles and procedures of Statistic.2nd ed. McGraw Hill International book Company, Tokyo, Japan
Stein WD (1962). Spermatozoa and enzyme induced dimmer formation and its role in the membrane permeability. The mechanism of movement of glycerol across the human Biochem. Biophys. Acta 59: 27 – 56
http://dx.doi.org/10.1016/0006-3002(62)90698-4
http://dx.doi.org/10.1016/0006-3002(62)90696-0
http://dx.doi.org/10.1016/0006-3002(62)90697-2
Swelum AA, Mansour HA, Elsayed AA, Amer HA (2011). Comparing ethylene glycol with glycerol for cryopreservation of buffalo bull semen in egg–yolk containing extenders. Theriogenol. 76: 833 – 842.
http://dx.doi.org/10.1016/j.theriogenology.2011.04.015
PMid:21664674
Younis M, Samad HA, Ahmad N, Ahmad I (1999). Fertility of frozen thawed semen collected from young, adult and old buffalo bulls during the low and the peak breeding seasons. Pak. Vet. J. 19: 78 – 80.