Advances in Animal and Veterinary Sciences
Research Article
Cutaneous Tumors in Dogs: A Retrospective Epidemiological and Histological Study of 112 Cases
Bardes B. Hassan1, Asmaa K. Al-Mokaddem1, Hisham A. Abdelrahman2, Ahmed Samir3, Mohamed R. Mousa1*
1Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt, Postal Code: 12211; 2Department of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt, Postal Code: 12211; 3Department of Microbiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt, Postal Code: 12211.
Abstract | Background: In the present study, a total of 112 dogs with cutaneous tumors were examined between November 2018 and November 2020. Out of 112 dogs, 118 skin tumor samples were obtained, of which 68 (57.63%) cases were malignant, and 50 (42.37%) cases were benign. Objectives: The objectives were to identify the most common histologic types of canine cutaneous tumors in Egypt, report the relative frequency of each tumor type, and elucidate the association of risk factors (age, sex, breed, and tumor anatomical site) with the development of common cutaneous tumor types. Methods: The samples were collected from different private veterinary practices. All samples were processed and diagnosed histopathologically in the Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt. Immunohistochemistry was used to confirm the diagnosed tumor types. Multinomial logistic regression models were used to study associations of the development of benign or malignant cutaneous tumors with risk factors. Results: Thirty different canine cutaneous tumors were diagnosed. Basal cell carcinoma (8.47%), papilloma (7.63%), infundibular keratinizing acanthoma (6.78%), liposarcoma (6.78%), and fibrosarcoma (5.93%) were the most common tumors. Tumors were commonly found on the trunk (25.42%), head (17.79%), and extremities (17.79%). The most affected breed was Golden Retriever (11.61%). The occurrence was predominant in males (53.57%) as compared to females (33.03%). Conclusion: The findings from this study are important for small animal clinical practices as they will serve as a useful reference to establish a preliminary diagnosis of cutaneous tumors in dogs as rapidly and precisely as possible.
Keywords | Anatomic site, Biopsy, Dog, Histopathology, Skin tumors
Received | August 02, 2021; Accepted | October 09, 2021; Published | December 13, 2021
*Correspondence | Mohamed R Mousa, Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt, Postal Code: 12211; Email: mohamed.refat@cu.edu.eg
Citation | Hassan BB, Al-Mokaddem AK, Abdelrahman HA, Samir A, Mousa MR (2022). Cutaneous tumors in dogs: a retrospective epidemiological and histological study of 112 cases. Adv. Anim. Vet. Sci. 10(1): 170-182.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.1.170.182
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2022 Mousa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cutaneous tumors commonly affect dogs (Graf et al., 2018) as the skin is significantly damaged by physical and chemical factors contributing to the development of canine cutaneous tumors (Pakhrin et al., 2007). Moreover, the skin has a large number of cells that are continuously renewed, rendering the skin more vulnerable to gene disorders with subsequent mutations (Tostes et al., 2017). Approximately 30% of all canine neoplasms arise in the skin (Kaldrymidou et al., 2002; Strafuss, 1985a). The incidence of canine cutaneous tumors is estimated to be 728 cases per 100,000 dogs per year (Kaldrymidou et al., 2002). Similar histological features of some cutaneous tumors result in confusing pathological diagnoses (Fuertes et al., 2013). An accurate diagnosis of the origin and type of neoplastic cells is important for identification, early treatment, and predicting the outcomes of many skin tumors (Araújo et al., 2012; Fuertes et al., 2013). Immunohistochemistry (IHC) is an important ancillary technique to confirm the diagnosis of tumors and determine the differentiation of neoplastic cells (Fuertes et al., 2013; Ramos-Vara et al., 2008). The most specific and useful markers for characterization of canine cutaneous tumors are antibodies against cytokeratin and vimentin proteins (Kozaki et al., 2001; Painter et al., 2010). Both cytokeratin and vimentin markers are used for distinguishing epithelial from mesenchymal differentiation (Painter et al., 2010).
Several retrospective studies have been performed to study the epidemiology of cutaneous tumors in dogs (Graf et al., 2018; Kok et al., 2019; Moraes et al., 2009). These studies differ considerably concerning the data collection and source, the sample numbers, study population, the size of the geographic area evaluated, and the quality of the result analysis (Graf et al., 2018; Kok et al., 2019). Some studies calculated either the relative tumor incidence or odds ratio (OR) based on the total patient data in the sample from the national canine cancer registries and veterinary authorities (Bronden et al., 2010; Gruntzig et al., 2016; Gruntzig et al., 2015). Others reported tumor incidence rates based on information utilized from insurance data (Dobson et al., 2002), diagnostic laboratories, and local animal registration data (Merlo et al., 2008). Although studies of canine cutaneous tumors from different geographic regions show similarities, there have also been reports of variations in tumor types and relative incidence. In other words, both environmental influences and dog breeds play a role in developing certain types of cutaneous tumors in relevant locations (Chikweto et al., 2011). For example, ultraviolet sunlight radiation has been involved as a risk factor for canine hemangioma, hemangiosarcoma, and squamous cell carcinoma (Hargis et al., 1992; Nikula et al., 1992).
It is worth noting that to date, no studies on canine skin tumor frequencies have been conducted in Cairo or Giza in Egypt. We hypothesized that the types and frequencies of canine skin tumors in Egypt are different from those reported elsewhere. In this study, we present an inclusive study of 118 canine skin tumors, collected from different private small animal clinical practices between November 2018 and November 2020. The objectives of this study were to characterize the most common histologic types of canine cutaneous tumors in Egypt, report the relative frequency of each tumor type, and investigate the relationship between such frequencies and the age, sex, breed, and tumor anatomical site of commonly diagnosed tumor types.
Materials and Methods
Study Population
The population used for this study included tissue biopsy and surgical excision cases recorded from 112 dogs between November 2018 and November 2020. The skin tumor samples were provided by various private veterinary practices in Cairo and Giza governorates in Egypt and were submitted to Leptovet laboratory (a private veterinary diagnostic laboratory) for further processing. The histopathological examination of skin tumor samples was conducted in the Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt. Clinical data including age, sex, breed, and tumor anatomic site were recorded and their influence on the frequency of skin tumor development was statistically determined. Crossbreed dogs were classed as mixed breed. All cases of skin tumors were included in the present study, even if any of the clinical data was missing. Cases with unidentified parameters (e.g. breed, age…) were listed as ‘not reported’. Cases with a diagnosis of hyperplastic lesions and epithelial cysts were excluded. Perianal (hepatoid) gland tumors, hamartomas, sebaceous and apocrine tumors were included. A tumor of the same type that occurs at more than one anatomical site simultaneously was considered as a single tumor event. Different tumor types that simultaneously occur and tumor recurrence in one patient were considered as multiple separate events (Graf et al., 2018; Kok et al., 2019). Tumors recorded on head, ear, eyelid, face, and nostril were considered as ‘head’. Tumors recorded on trunk, chest, flank, pelvis, back, abdomen, mammary gland, and perianal were considered as ‘trunk’. Tumors recorded on vagina, scrotum, and penis were considered as genitalia. Tumors were recorded on forelimb, hindlimb, digit, foot, pad, and paw were considered as ‘extremities’. Anatomical sites were listed as head, neck, trunk, extremities, and genitalia.
Tumor Diagnosis and Classification
Tumor cases were diagnosed by two independent veterinary pathologists. The samples were fixed in 10% neutral buffered formalin, processed, sectioned at 4 µm, and stained with hematoxylin and eosin (HE). The histopathological examination was performed under Olympus BX43 microscope, Tokyo, Japan. The images were captured using Olympus DP 27 camera, Tokyo, Japan. Tumor subtypes were grouped into main types of tumors according to the World Health Organization International Histological Classification of Tumors of Domestic Animals (Goldschmidt et al., 2007). Mast cell tumors, transmissible venereal tumors, and peripheral nerve sheath tumors were considered as malignant tumors. The frequencies of benign and malignant tumors were recorded. Mast cell tumors were stained with a special stain (toluidine blue).
Table 1: Immunohistochemical antibody panel applied to some cases of the canine skin tumors.
| Antibody |
Type (catalog number) |
Source | Dilution | Antigen retrieval | Positive expression |
| CD3 |
Rabbit polyclonal antibody (103A-74)
|
Merck KGaA, Darmstadt, Germany | 1:200 | Antigen retrieval was done in the pre-treatment (PT) module, PT Link (Dako, Denmark). Briefly, tissue sections were subjected to 95 °C for 40 min. in Epitope Retrieval Solution (Dako) in accordance with manufacturer’s instruction for HercepTest (Dako). | Lymphoma |
| Glial fibrillary acidic protein (GFAP) | Rabbit polyclonal antibody (PA1-10019) | Thermo Fisher Scientific, Freemont, CA, USA | 1:1000 | Peripheral nerve sheath tumor | |
| Vimentin (V9) |
Mouse monoclonal antibody (347M-14)
|
Merck KGaA, Darmstadt, Germany | 1:200 | Soft tissue sarcoma | |
| Vascular endothelial growth factor (VEGF) | Mouse monoclonal antibody (sc-7269) | Santa Cruz Biotechnology, Inc. | 1:100 | Hemangiosarcoma | |
| Cytokeratin 14 (CK14) | Rabbit monoclonal [SP53] (ab119695) | Abcam, Cambridge, UK | 1:100 |
Adenoma and adenocarcinoma |
Table 2: Distributions of the 112 dogs with cutaneous tumors recorded in Egypt between 2018 and 2020 according to number of tumors per dog, examination year, and dog breed, and the respective age (minimum [min], maximum [max], mean, and standard error [SE]) and sex (numbers and ratio).
| N | % | Age (years) | Sex | ||||||||
|
NRa |
mean ± SE | min – max |
NRa |
Male | Female |
M:Fb |
|||||
| All dogs | 112 | 100 | 13 | 6.85 ± 0.31 |
0.5 – 17.0 |
15 | 60 | 37 | 1.6:1 | ||
| Dogs with one tumor | 106 | 94.64 | 94 | 6.95 ± 0.33 | 0.5 – 17.0 | 14 | 56 | 36 | 1.6:1 | ||
| Dogs with two tumors | 6 | 5.36 | 5 | 5.00 ± 0.55 | 4.0 – 7.0 | 1 | 4 | 1 |
4:1 |
||
| By year | |||||||||||
| 2018 | 4 | 3.57 | 2 | 9.00 ± 1.00 | 8.0 – 10.0 | 2 | 1 | 1 | 1:1 | ||
| 2019 | 30 | 26.79 | 9 | 6.51 ± 0.65 | 0.8 – 11.0 | 7 | 12 | 11 | 1.1:1 | ||
| 2020 | 78 |
69.64 |
2 | 6.89 ± 0.37 | 0.5 – 17.0 | 6 | 47 | 25 | 1.9:1 | ||
| By breed | |||||||||||
| Not reported | 30 | 26.79 | 11 | 6.30 ± 0.68 | 0.8 – 11.0 | 6 | 11 | 13 | 0.8:1 | ||
| Golden Retriever | 13 | 11.61 | 0 | 7.85 ± 0.80 |
4.0 – 12.0 |
1 | 8 | 4 | 2:1 | ||
| German Shepherd | 12 | 10.71 | 0 | 5.18 ± 0.80 | 0.5 – 9.0 | 1 | 7 | 4 | 1.8:1 | ||
| Labrador Retriever | 8 | 7.14 | 0 | 6.88 ± 0.79 | 3.0 – 11.0 | 1 | 5 | 2 |
2.5:1 |
||
| Mixed Breed | 7 | 6.25 | 0 | 5.86 ± 0.86 | 3.0 – 10.0 | 0 | 6 | 1 | 6:1 | ||
| Rottweiler | 7 | 6.25 | 0 | 7.00 ± 0.58 | 5.0 – 9.0 | 0 | 6 | 1 | 6:1 | ||
| Baladi | 5 | 4.46 | 1 | 4.92 ± 1.70 |
0.7 – 9.0 |
1 | 2 | 2 | 1:1 | ||
| Griffon | 5 | 4.46 | 1 | 4.75 ± 1.70 | 2.0 – 9.0 | 1 | 2 | 2 | 1:1 | ||
| Pit Bull | 3 | 2.68 | 0 | 8.33 ± 0.33 | 8.0 – 9.0 | 0 | 2 | 1 |
2:1 |
||
| Cane Corso | 2 | 1.79 | 0 | 3.00 ± 1.00 | 2.0 – 4.0 | 0 | 1 | 1 | 1:1 | ||
| Cocker Spaniel | 2 | 1.79 | 0 | 13.00 ± 2.00 | 11.0 – 15.0 | 1 | 1 | 0 | 1:0 | ||
| Dachshund | 2 | 1.79 | 0 | 12.50 ± 0.50 |
12.0 – 13.0 |
0 | 0 | 2 | 0:2 | ||
| Doberman | 2 | 1.79 | 0 | 7.00 ± 0.00 | 7.0 – 7.0 | 0 | 1 | 1 | 1:1 | ||
| Pug | 2 | 1.79 | 0 | 12.50 ± 4.50 | 8.0 – 17.0 | 1 | 1 | 0 |
1:0 |
||
| Siberian Husky | 2 | 1.79 | 0 | 7.50 ± 0.50 | 7.0 – 8.0 | 0 | 2 | 0 | 2:0 | ||
| Alaskan Malamute | 1 | 0.89 | 0 | 5.00 ± 0.00 | 5.0 – 5.0 | 0 | 0 | 1 | 0:1 | ||
| American Bully | 1 | 0.89 | 0 | 8.00 ± 0.00 |
8.0 – 8.0 |
0 | 0 | 1 | 0:1 | ||
| Beagle | 1 | 0.89 | 0 | 4.00 ± 0.00 | 4.0 – 4.0 | 0 | 1 | 0 | 1:0 | ||
| Dalmatian | 1 | 0.89 | 0 | 9.00 ± 0.00 | 9.0 – 9.0 | 0 | 1 | 0 |
1:0 |
||
| French Bulldog | 1 | 0.89 | 0 | 2.00 ± 0.00 | 2.0 – 2.0 | 1 | 0 | 0 | - | ||
| Great Dane | 1 | 0.89 | 0 | 8.00 ± 0.00 | 8.0 – 8.0 | 1 | 0 | 0 | - | ||
| Italian Grey Hound | 1 | 0.89 | 0 | 9.00 ± 0.00 |
9.0 – 9.0 |
0 | 0 | 1 | 0:1 | ||
| Pekingese | 1 | 0.89 | 0 | 11.00 ± 0.00 | 11.0 – 11.0 | 0 | 1 | 0 | 1:0 | ||
| Ridgeback | 1 | 0.89 | 0 | 8.00 ± 0.00 | 8.0 – 8.0 | 0 | 1 | 0 |
1:0 |
||
| Schnoodle | 1 | 0.89 | 0 | 11.00 ± 0.00 | 11.0 – 11.0 | 0 | 1 | 0 | 1:0 | ||
|
a NR: not reported; b M: Male; F: Female |
|||||||||||
Table 3: Tumor histopathological diagnoses, relative frequency and the respective distribution of anatomical site of the 118 canine cutaneous tumors recorded in Egypt between 2018 and 2020.
| Tumor Type | N | % | Anatomical Site | |||||||
|
NRa |
Trunk | Extremities | Head | Genitalia | Neck | |||||
| All tumors | 118 | 100 | 36 | 30 | 21 | 21 | 6 | 4 | ||
| Benign Tumors | 50 | 42.37 | 22 | 10 | 7 | 8 | 0 | 3 | ||
| Malignant Tumors | 68 | 57.63 | 14 | 20 | 14 | 13 | 6 | 1 | ||
| Epithelial Tumors | 56 | 47.46 | 17 | 15 | 4 | 17 | 0 |
3 |
||
| Epidermal Tumors | 24 | 20.34 | 8 | 3 | 3 | 10 | 0 | 0 | ||
| Papilloma | 9 | 7.63 | 5 | 0 | 1 | 3 | 0 | 0 | ||
| Squamous Cell Carcinoma | 5 | 4.24 | 1 | 1 | 2 | 1 | 0 | 0 | ||
| Basal Cell Carcinoma | 10 | 8.47 | 2 | 2 | 0 | 6 | 0 | 0 | ||
| Hair Follicle Tumors | 14 | 11.86 | 8 | 3 | 1 | 0 | 0 | 2 | ||
| Benign Trichoepithelioma | 4 | 3.39 | 2 | 0 | 1 | 0 | 0 | 1 | ||
| Malignant Trichoepithelioma | 2 | 1.69 | 2 | 0 | 0 | 0 | 0 | 0 | ||
| Infundibular Keratinizing Acanthoma | 8 | 6.78 | 4 | 3 | 0 | 0 | 0 | 1 | ||
| Sebaceous Gland Tumors | 15 |
12.71 |
1 | 9 | 0 | 5 | 0 | 0 | ||
| Sebaceous Gland Adenoma | 1 | 0.85 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| Sebaceous Gland Epithelioma | 4 | 3.39 | 0 | 1 | 0 | 3 | 0 | 0 | ||
| Hepatoid Gland Adenoma | 3 | 2.54 | 0 | 3 | 0 | 0 | 0 | 0 | ||
| Hepatoid Gland Adenocarcinoma | 5 | 4.24 | 0 | 5 | 0 | 0 | 0 | 0 | ||
| Meibomian Adenocarcinoma | 2 |
1.69 |
0 | 0 | 0 | 2 | 0 | 0 | ||
| Apocrine Glands Tumors | 3 | 2.54 | 0 | 0 | 0 | 2 | 0 | 1 | ||
| Apocrine Gland Adenoma | 1 | 0.85 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Ceruminous Gland Adenoma | 1 | 0.85 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| Ceruminous Gland Adenocarcinoma | 1 | 0.85 | 0 | 0 | 0 | 1 | 0 |
0 |
||
| Mesenchymal Tumors | 41 | 34.75 | 16 | 13 | 10 | 0 | 1 | 1 | ||
| Benign Soft Tissue Tumors | 9 | 7.63 | 5 | 3 | 1 | 0 | 0 | 0 | ||
| Lipoma | 6 | 5.08 | 3 | 3 | 0 | 0 | 0 | 0 | ||
| Fibroma | 3 | 2.54 | 2 | 0 | 1 | 0 | 0 |
0 |
||
| Soft Tissue Sarcomas | 21 | 17.80 | 6 | 8 | 6 | 0 | 0 | 1 | ||
| Fibrosarcoma | 7 | 5.93 | 3 | 2 | 1 | 0 | 0 | 1 | ||
| Malignant Peripheral Nerve Sheath Tumor | 5 | 4.24 | 1 | 0 | 4 | 0 | 0 | 0 | ||
| Liposarcoma | 8 | 6.78 | 2 | 6 | 0 | 0 | 0 | 0 | ||
| Myxosarcoma | 1 |
0.85 |
0 | 0 | 1 | 0 | 0 | 0 | ||
| Vascular Tumors | 6 | 5.08 | 4 | 0 | 1 | 0 | 1 | 0 | ||
| Hemangioma | 2 | 1.69 | 2 | 0 | 0 | 0 | 0 | 0 | ||
| Hemangiosarcoma | 4 | 3.39 | 2 | 0 | 1 | 0 | 1 | 0 | ||
| Rhabdomyosarcoma | 5 |
4.24 |
1 | 2 | 2 | 0 | 0 | 0 | ||
| Round Cell Tumors | 9 | 7.63 | 1 | 2 | 4 | 2 | 0 | 0 | ||
| Epitheliotropic T-cell Lymphoma | 1 | 0.85 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| Mast Cell Tumor | 4 | 3.39 | 0 | 2 | 2 | 0 | 0 | 0 | ||
| Histiocytoma | 4 | 3.39 | 1 | 0 | 2 | 1 | 0 |
0 |
||
| Melanocytic Tumors | 5 | 4.24 | 1 | 0 | 2 | 2 | 0 | 0 | ||
| Melanocytoma | 2 | 1.69 | 1 | 0 | 1 | 0 | 0 | 0 | ||
| Malignant Melanoma | 3 | 2.54 | 0 | 0 | 1 | 2 | 0 | 0 | ||
| Transmissible Venereal Tumors | 5 | 4.24 | 0 | 0 | 0 | 0 | 5 | 0 | ||
| Hamartoma | 2 | 1.69 | 1 | 0 | 1 | 0 | 0 |
0 |
||
|
a NR: not reported |
||||||||||
Table 4: Tumor histopathological diagnoses of the 118 canine cutaneous tumors recorded in Egypt between 2018 and 2020; and the respective age (minimum [min], maximum [max], mean, and standard error [SE]) and sex (numbers and ratio).
| Tumor Type | N | Age (years) | Sex | |||||||
|
NRa |
mean ± SE | min – max |
NRa |
Male | Female |
M:Fb |
||||
| All tumors | 118 | 14 | 6.77 ± 0.30 | 0.5 – 17.0 | 16 | 64 | 38 | 1.7:1 | ||
| Benign Tumors | 50 | 6 | 6.06 ± 0.45 | 0.5 – 12.0 | 7 | 27 | 16 | 1.7:1 | ||
| Malignant Tumors | 68 | 8 | 7.28 ± 0.40 | 0.8 – 17.0 | 9 | 37 | 22 | 1.7:1 | ||
| Epithelial Tumors | 56 | 9 | 6.73 ± 0.47 | 0.7 – 17.0 | 10 | 31 | 15 | 2.1:1 | ||
| Epidermal Tumors | 24 | 4 | 5.97 ± 0.66 | 0.7 – 12.0 | 4 | 16 | 4 | 4:1 | ||
| Papilloma | 9 | 1 | 3.67 ± 0.67 | 0.7 – 5.0 | 1 | 7 | 1 | 7:1 | ||
| Squamous Cell Carcinoma | 5 | 1 | 8.75 ± 1.11 | 7.0 – 12.0 | 0 | 3 | 2 | 1.5:1 | ||
| Basal Cell Carcinoma | 10 | 2 | 6.88 ± 0.91 | 4.0 – 11.0 | 3 | 6 | 1 | 6:1 | ||
| Hair Follicle Tumors | 14 | 1 | 5.23 ± 0.70 |
1.0 – 11.0 |
1 | 5 | 8 | 1:1.6 | ||
| Benign Trichoepithelioma | 4 | 0 | 6.75 ± 1.44 | 5.0 – 11.0 | 0 | 1 | 3 | 1:3 | ||
| Malignant Trichoepithelioma | 2 | 0 | 6.50 ± 0.50 | 6.0 – 7.0 | 0 | 0 | 2 | 0:2 | ||
| Infundibular Keratinizing Acanthoma | 8 | 1 | 4.00 ± 0.82 | 1.0 – 7.0 | 1 | 4 | 3 | 1.3:1 | ||
| Sebaceous Gland Tumors | 15 | 3 | 9.67 ± 0.79 | 6.0 – 17.0 | 3 | 10 | 2 | 5:1 | ||
| Sebaceous Gland Adenoma | 1 | 0 | 8.00 ± 0.00 | 8.0 – 8.0 | 1 | 0 | 0 | - | ||
| Sebaceous Gland Epithelioma | 4 | 1 | 9.67 ± 1.20 | 8.0 – 12.0 | 1 | 3 | 0 | 3:0 | ||
| Hepatoid Gland Adenoma | 3 | 0 | 9.00 ± 0.58 | 8.0 – 10.0 | 0 | 3 | 0 | 3:0 | ||
| Hepatoid Gland Adenocarcinoma | 5 | 2 | 11.00 ± 3.21 | 6.0 – 17.0 | 1 | 3 | 1 | 3:1 | ||
| Meibomian Adenocarcinoma | 2 | 0 | 9.50 ± 0.50 | 9.0 – 10.0 | 0 | 1 | 1 | 1:1 | ||
| Apocrine Glands Tumors | 3 | 1 | 6.50 ± 0.50 | 6.0 – 7.0 | 2 | 0 | 1 | 0:1 | ||
| Apocrine Gland Adenoma | 1 | 0 | 7.00 ± 0.00 | 7.0 – 7.0 | 0 | 0 | 1 | 0:1 | ||
| Ceruminous Gland Adenoma | 1 | 1 | - | - | 1 | 0 | 0 | - | ||
| Ceruminous Gland Adenocarcinoma | 1 | 0 | 6.00 ± 0.00 | 6.0 – 6.0 | 1 | 0 | 0 | - | ||
| Mesenchymal Tumors | 41 | 3 | 7.16 ± 0.48 | 0.5 – 15.0 | 6 | 20 | 15 | 1.3:1 | ||
| Benign Soft Tissue Tumors | 9 | 2 | 7.21 ± 1.13 | 0.5 – 9.0 | 2 | 4 | 3 | 1.3:1 | ||
| Lipoma | 6 | 1 | 6.70 ± 1.56 | 0.5 – 9.0 | 2 | 1 | 3 | 1:3 | ||
| Fibroma | 3 | 1 | 8.50 ± 0.50 | 8.0 – 9.0 | 0 | 3 | 0 | 3:0 | ||
| Soft Tissue Sarcomas | 21 | 1 | 7.34 ± 0.73 | 0.8 – 15.0 | 3 | 10 | 8 | 1.3:1 | ||
| Fibrosarcoma | 7 | 1 | 7.17 ± 1.33 |
2.0 – 12.0 |
2 | 2 | 3 | 1:1.5 | ||
| Malignant Peripheral Nerve Sheath Tumor | 5 | 0 | 10.2 ± 1.53 | 6.0 – 15.0 | 1 | 3 | 1 | 3:1 | ||
| Liposarcoma | 8 | 0 | 5.97 ± 0.87 | 0.8 – 9.0 | 0 | 5 | 3 | 1.7:1 | ||
| Myxosarcoma | 1 | 0 | 5.00 ± 0.00 | 5.0 – 5.0 | 0 | 0 | 1 | 0:1 | ||
| Vascular Tumors | 6 | 0 | 8.00 ± 0.97 | 4.0 – 11.0 | 1 | 2 | 3 | 1:1.5 | ||
| Hemangioma | 2 | 0 | 8.00 ± 1.00 | 7.0 – 9.0 | 0 | 0 | 2 | 0:2 | ||
| Hemangiosarcoma | 4 | 0 | 8.00 ± 1.47 | 4.0 – 11.0 | 1 | 2 | 1 | 2:1 | ||
| Rhabdomyosarcoma | 5 | 0 | 5.40 ± 0.87 |
2.0 – 7.0 |
0 | 4 | 1 | 4:1 | ||
| Round Cell Tumors | 9 | 1 | 4.88 ± 1.01 | 1.0 – 9.0 | 0 | 5 | 4 | 1.3:1 | ||
| Epitheliotropic T-cell Lymphoma | 1 | 0 | 6.00 ± 0.00 | 6.0 – 6.0 | 0 | 1 | 0 | 1:0 | ||
| Mast Cell Tumor | 4 | 1 | 6.00 ± 2.08 | 2.0 – 9.0 | 0 | 2 | 2 | 1:1 | ||
| Histiocytoma | 4 | 0 | 3.75 ± 1.31 | 1.0 – 6.0 | 0 | 2 | 2 | 1:1 | ||
| Melanocytic Tumors | 5 | 0 | 9.00 ± 1.41 | 5.0 – 13.0 | 0 | 2 | 3 | 1:1.5 | ||
| Melanocytoma | 2 | 0 | 8.00 ± 3.00 | 5.0 – 11.0 | 0 | 1 | 1 | 1:1 | ||
| Malignant Melanoma | 3 | 0 | 9.67 ± 1.76 |
7.0 – 13.0 |
0 | 1 | 2 | 1:2 | ||
| Transmissible Venereal Tumors | 5 | 1 | 4.25 ± 0.63 | 3.0 – 6.0 | 0 | 4 | 1 | 4:1 | ||
| Hamartoma | 2 | 0 | 7.00 ± 0.00 | 7.0 – 7.0 | 0 | 2 | 0 | 2:0 | ||
|
a NR: not reported; b M: Male; F: Female |
||||||||||
Table 5: Odds ratio (OR), 95% confidence intervals (95% CI) for OR, and significance statistics of the multinomial logistic regression models evaluating the association of benign and malignant cutaneous tumor development with dog breed. Odds ratio was statistically significant if the null value (OR = 1.0) is not contained within the 95% CI. Regression models were statistically significant at P < 0.05 if (bold*).
| Dog Breed | Odds Ratio | Significance | |||||||
| Benign Tumors | Malignant Tumors |
2(1) |
P-value |
||||||
| Estimate | 95% CI | Estimate | 95% CI | ||||||
| lower | upper | lower | upper | ||||||
| Baladi | 1 | - | - | 1 | - | - | - | - | |
| Beagle | >999.999 | <0.001 | >999.999 | <0.001 | <0.001 | >999.999 | 0.0006 | 0.9798 | |
| Cane Corso | 1.00 | 0.063 | 15.988 | 1.00 | 0.063 | 15.988 | <0.0001 | >0.999 | |
| Cocker Spaniel | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0003 | 0.9857 | |
| Dachshund | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0003 | 0.9857 | |
| Dalmatian | >999.999 | <0.001 | >999.999 | <0.001 | <0.001 | >999.999 | 0.0003 | 0.9857 | |
| Doberman | 1.00 | 0.063 | 15.988 | 1.00 | 0.063 | 15.988 | <0.0001 | >0.999 | |
| German Shepherd | 0.33 | 0.067 | 1.652 | 3.00 | 0.606 | 14.864 | 1.8104 | 0.1785 | |
| Golden Retriever | 0.25 | 0.053 | 1.177 | 4.00 | 0.849 | 18.836 | 3.0749 | 0.0795 | |
| Griffon | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0010 | 0.9752 | |
| Italian Grey Hound | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0003 | 0.9857 | |
| Labrador Retriever | 0.20 | 0.023 | 1.712 | 5.00 | 0.584 | 42.797 | 2.1586 | 0.1418 | |
| Mixed Breed | 1.50 | 0.251 | 8.977 | 0.667 | 0.111 | 3.99 | 0.1973 | 0.6569 | |
| Pekingese | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0003 | 0.9857 | |
| Pit Bull | >999.999 | <0.001 | >999.999 | <0.001 | <0.001 | >999.999 | 0.0003 | 0.9857 | |
| Pug | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0003 | 0.9857 | |
| Ridgeback | >999.999 | <0.001 | >999.999 | <0.001 | <0.001 | >999.999 | 0.0003 | 0.9857 | |
| Rottweiler | 1.00 | 0.141 | 7.099 | 1.00 | 0.141 | 7.099 | <0.0001 | >0.999 | |
| Siberian Husky | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0006 |
0.9798 |
|
Table 6: Odds ratio (OR), 95% confidence intervals (95% CI) for OR, and significance statistics of the multinomial logistic regression models evaluating the association of benign and malignant cutaneous tumor development with dog sex, dog age, and anatomical site of tumor. Odds ratio was statistically significant if the null value (OR = 1.0) is not contained within the 95% CI. Regression models were statistically significant at P < 0.05 if (bold*).
| Variable | Odds Ratio | Significance | ||||||||
| Benign Tumors | Malignant Tumors |
P-value |
||||||||
| Estimate | 95% CI | Estimate | 95% CI | |||||||
| lower | upper | lower | upper | |||||||
| Sex | ||||||||||
| Male | 1 | - | - | 1 | - | - | - | - | ||
| Female | 0.545 | 0.202 | 1.475 | 1.833 | 0.678 | 4.957 | 1.4262 | 0.2324 | ||
| Site | ||||||||||
| Head | 1 | - | - | 1 | ||||||
| Extremities | 0.308 | 0.10 | 0.944 | 3.25 | 1.06 | 9.967 | 4.2494 | 0.0393* | ||
| Genitalia | <0.001 | <0.001 |
>999.999 |
>999.999 | <0.001 | >999.999 | 0.0013 | 0.9714 | ||
| Neck | >999.999 | <0.001 | >999.999 | <0.001 | <0.001 | >999.999 | 0.0006 | 0.9798 | ||
| Trunk | 0.636 | 0.247 | 1.642 | 1.571 | 0.609 | 4.054 | 0.8739 |
0.3499 |
||
| Age (years) | ||||||||||
| ≤ 1 | 1 | - | - | 1 | - | - | - | - | ||
| 2 | 0.50 | 0.045 | 5.514 | 2.00 | 0.181 | 22.056 | 0.3203 | 0.5714 | ||
| 3 | 1.00 | 0.063 | 15.988 | 1.00 | 0.063 | 15.988 | <0.0001 | >0.999 | ||
| 4 | 1.00 | 0.202 | 4.955 | 1.00 | 0.202 | 4.955 | <0.0001 | >0.999 | ||
| 5 | 0.17 | 0.02 | 1.384 | 6.00 | 0.722 | 49.837 | 2.7518 | 0.0971 | ||
| 6 | 0.17 | 0.02 | 1.384 | 6.00 | 0.722 | 49.837 | 2.7518 | 0.0971 | ||
| 7 | 0.17 | 0.02 | 1.384 | 6.00 | 0.722 | 49.837 | 2.7518 | 0.0971 | ||
| 8 | 2.00 | 0.366 | 10.919 | 0.50 | 0.092 | 2.73 | 0.6406 | 0.4235 | ||
| 9 | 0.75 | 0.168 | 3.351 | 1.33 | 0.298 | 5.957 | 0.1419 | 0.7064 | ||
| 10 | 0.50 | 0.045 | 5.514 | 2.00 | 0.181 | 22.056 | 0.3203 | 0.5714 | ||
| 11 | 0.50 | 0.045 | 5.514 | 2.00 | 0.181 | 22.056 | 0.3203 | 0.5714 | ||
| 12 | 1.00 | 0.063 |
15.988 |
1.00 | 0.063 | 15.988 | <0.0001 | >0.999 | ||
| 13 | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0003 | 0.9857 | ||
| 17 | <0.001 | <0.001 | >999.999 | >999.999 | <0.001 | >999.999 | 0.0003 |
0.9857 |
||
Immunohistochemistry (IHC)
Immunohistocemistry was performed to confirm the histopathological diagnosis of specific tumor types. Immunohistochemical antibodies used in tumor diagnosis are summarized in Table 1.
Statistical Analysis
The relative frequency (percentage of all cutaneous tumor types) and the OR with a 95% confidence interval (95% CI) were calculated. To assess the association of dog sex, age, breed, and tumor anatomical site with the development of benign and malignant cutaneous tumors, two multinomial logistic regression models, integrated with likelihood ratio test and Pearson’s chi-square (x2) test, were performed. The dependent variable in first model was defined as benign cutaneous tumor development (either yes or no) while in second model was defined as malignant cutaneous tumor development (either yes or no). In both models, the independent variables were defined as sex (reference = male), age (reference = less than or equal to 1 year), breed (reference = Baladi), and anatomical site of tumor (reference = head). Data management and statistical analyses were performed using SAS® version 9.4 (SAS, 2013). Statistical significance was set at P < 0.05, and all data were presented as the mean ± standard error of the mean (SE).
Results
Canine Population in the Database
From November 2018 to November 2020, tissues from 112 dogs were collected by biopsy and surgical excision. Out of this database, 118 samples from 112 dogs were diagnosed with a skin tumor. Five dogs had tumor recurrence and one dog had two different tumors in two different sites.
Breed Distribution
The dog breed was not reported in 30 dogs (26.79%). The remaining dog cases (n = 82 dogs) included 24 different dog breeds. The five most common dog breeds were Golden Retriever (n = 13; 11.61%), German Shepherd (n = 12; 10.71%), Labrador Retriever (n = 8; 7.14%), Mixed breed (n = 7; 6.25%), and Rottweiler (n = 7; 6.25%).
Sex Distribution
The sex of the dog was not reported for 15 dogs (13.39%). Most tumors samples were submitted from males (n = 60; 53.57%) as compared to females (n = 37; 33.03%).
Age Distribution
Dog age was not reported in 13 cases (11.60%). The reported age of dog cases in this study ranged from 6 months to 17 years (mean = 6.85 years; SE = 0.31). More than half of the dogs were between 5 and 9 years of age (n = 65; 58.03%). Age distribution breakdown is summarized in Figure 1.
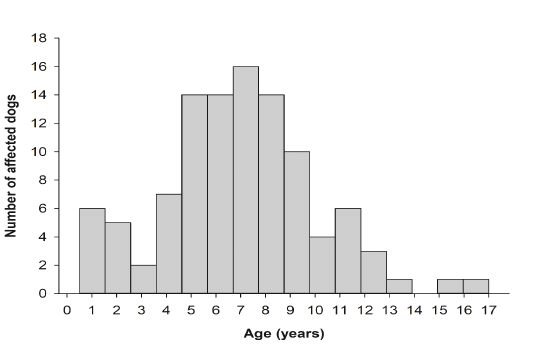
Figure 1: Number of dogs reported in the database in relation to age. More than half of the dog populations were between 5 and 9 years of age.
Tumor Types and Anatomic Distribution
Tumor histopathological diagnoses, relative frequency, and the respective distribution of dog breed, age, sex, and tumor anatomical site of the 118 canine cutaneous tumors diagnosed during the 2-year study period are shown in Tables 2-4. Of 118 cutaneous tumors, 68 (57.63%) were malignant and 50 (42.37%) were benign. The five most frequently diagnosed tumors were basal cell carcinoma (n = 10; 8.47%), papilloma (n = 9; 7.63%), infundibular keratinizing acanthoma (n = 8; 6.78%), liposarcoma (n= 8; 6.78%) and fibrosarcoma (n = 7; 5.93%). These tumors comprised 35.59% of the total cutaneous tumor cases. Of the 118 tumor samples 21 (17.79%) were localized on the head, 4 (3.38%) on the neck, 30 (25.42%) on the trunk, 6 (5.08%) on the genital organs, and 21 (17.79%) on the extremities. For 36 tumors (30.50%), the location on the skin was not reported.
Multinomial Logistic Regression Analysis
The results from the multiple logistic regression analyses are shown in Tables 5 and 6. There was no association between dog breed and cutaneous tumor development (x2(18) = 23.47, P = 0.1733; Table 5). Cutaneous tumor development was not associated with dog sex (x2(1) = 1.49, P = 0.2218) nor dog age (x2(13) = 16.50, P = 0.2231). However, it was associated with tumor anatomical site (x2(4) = 714.23, P = 0.0066; Table 6). Malignant cutaneous tumors in dog extremities had a significantly increased odds ratio compared to malignant cutaneous tumors in dog head (x2(1) = 4.25, P = 0.0393; Table 6).
Histopathological and Immunohistochemical Diagnoses of Tumor Samples
In the present study, 30 different tumor types have been diagnosed and classified based on their histopathological characteristics. Epidermal tumors consisted of papilloma, squamous cell carcinoma, and basal cell carcinoma (Figs. 2-4). Hair follicle tumors consisted of benign and malignant trichoepithelioma, and infundibular keratinizing acanthoma (Figs. 5-7). Sebaceous gland tumors consisted of sebaceous adenoma, sebaceous epithelioma, hepatoid gland adenoma (positive for cytokeratin [CK14+]), hepatoid gland adenocarcinoma (CK14+), and meibomian adenocarcinoma (Figs. 8-14). Apocrine gland tumors consisted of apocrine gland adenoma, ceruminous gland adenoma, and ceruminous gland adenocarcinoma (Figs. 15-17). Melanocytic tumors consisted of melanocytoma and malignant melanoma (Figs. 18 and 19). Benign soft tissue tumors consisted of lipoma and fibroma (Figs. 20 and 21). Soft tissue sarcomas consisted of liposarcoma, fibrosarcoma (positive for vimentin [V9+]), myxosarcoma, and malignant peripheral nerve sheath tumor (positive for glial fibrillary acidic protein [GFAP+]) (Figs. 22-27). Vascular tumors consisted of hemangioma and hemangiosarcoma (positive for vascular endothelial growth factor [VEGF+]) (Figs. 28-30). Rhabdomyosarcoma represented muscle tumors (Fig. 31). Round cell tumors consisted of lymphoma (positive for CD3+), mast cell tumor (inset: toluidine blue stain), and histiocytoma (Figs. 32-35). Hamartoma and transmissible venereal tumors are presented in Figs. 36 and 37.
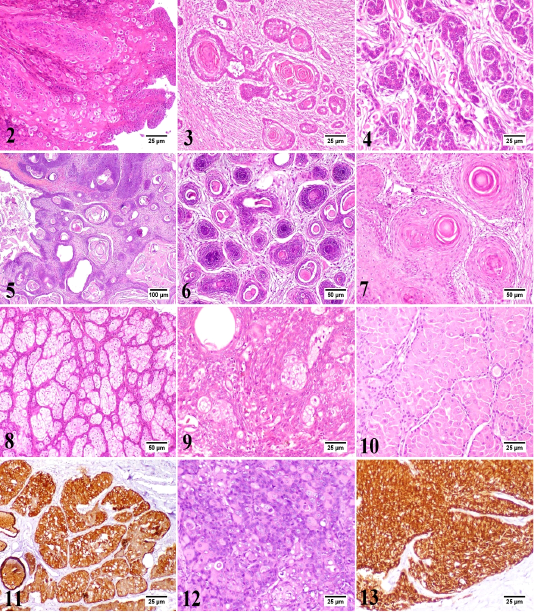
Figure 2-13: 2) Canine papilloma, skin, dog. The hyperplastic epidermal cells are forming inward projections into the underlying stroma with marked parakeratosis. Hematoxylin and Eosin (HE). 3) Canine squamous cell carcinoma, skin, dog. The neoplasm is a well-differentiated. The neoplastic cells are forming a distinct keratin “pearls”. HE. 4) Canine basal cell carcinoma, skin, dog. Basophilic neoplastic cells with hyperchromatic nuclei are arranged in cords or sheets. HE. 5) Canine benign trichoepithelioma (Cystic variant), skin, dog. Multiple small cysts filled with keratinous debris. HE. 6) Canine malignant trichoepithelioma, skin, dog. The hyperchromatic neoplastic cells are invading into the dermis with central area of necrosis. HE. 7) Canine infundibular keratinizing acanthoma, skin, dog. Multiple cysts consisted of central lamellated keratin and proliferating keratinocytes were noticed. HE. 8) Canine sebaceous adenoma, skin, dog. The proliferating neoplastic cells are arranged in lobules separated by the pre-existing collagen of dermis. HE. 9) Canine sebaceous epithelioma, skin, dog. There are numerous small basophilic reserve cells with few sebocytes and ducts. HE. 10) Canine hepatoid gland adenoma, perianal gland, dog. The neoplastic cells are arranged in islands or trabeculae resembling the normal gland. HE. 11) Canine hepatoid gland adenoma, perianal gland, dog. Most neoplastic cells show intense immunolabelling. Immunohistochemistry (IHC) for cytokeratin 14 (CK14). 12) Canine hepatoid gland adenocarcinoma, perianal gland, dog. Poorly differentiated neoplastic cells with hyperchromatic nuclei are arranged in clusters or irregular sheets. HE. 13) Canine hepatoid gland adenocarcinoma, perianal gland, dog. The neoplastic cells show intense immunolabelling. IHC for CK14.

Figure 14-25: 14) Canine meibomian adenocarcinoma, meibomian gland, dog. The neoplastic cells are grouped in lobules with presence of intracytoplasmic lipid vacuoles of variable sizes. HE. 15) Canine apocrine gland adenoma, skin, dog. The gland is lined by simple cuboidal cells with secretory blebs on the luminal surface. HE. 16) Canine ceruminous gland adenoma, skin, dog. The tumor resembles the normal gland with retention of intraluminal material. HE. 17) Canine ceruminous gland carcinoma, skin, dog. The neoplastic cells are arranged in clusters with marked pleomorphism. HE. 18) Canine melanocytoma, skin, dog. The neoplastic melanocytes are present at the lower layers of the epidermis and just beneath the epidermis either as single cells or as small clusters. HE. 19) Canine malignant melanoma, skin, dog. The neoplastic cells are showing variable intracytoplasmic content of melanin pigment infiltrating the superficial dermis. HE. 20) Canine lipoma, skin, dog. The neoplastic cells are vacuolated, variable sized and are arranged in lobules with fine fibrous separating septa. HE. 21) Canine fibroma, skin, dog. The tumor is consisted of interwoven bundles of collagen. HE. 22) Canine liposarcoma, skin, dog. The neoplastic cells have variable sized fat vacuoles with marked pleomorphism. HE. 23) Canine fibrosarcoma, skin, dog. The neoplastic cells are pleomorphic and forming interlacing fascicles with little deposition of collagenous matrix. HE. 24) Canine fibrosarcoma, skin, dog. The neoplastic cells show intense immunolabelling. IHC for vimentin. 25) Canine myosarcoma, skin, dog. The neoplastic stellate and spindle-shaped cells are suspended in faint blue myxomatous matrix. HE.
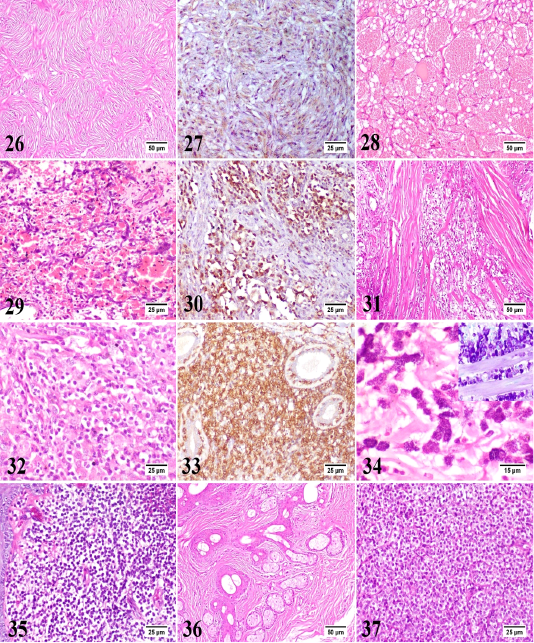 Figure 26-37: 26) Canine peripheral nerve sheath tumor, skin, dog. The spindle neoplastic cells are arranged in wavy bundles, palisades and whorls. HE. 27) Canine peripheral nerve sheath tumor, skin, dog. The neoplastic cells show moderate immunolabelling. IHC for glial fibrillary acidic protein (GFAP). 28) Canine hemangioma, skin, dog. The tumor is formed of variably sized blood spaces containing erythrocytes and lined by a single layer of uniform endothelial cells. HE. 29) Canine hemangiosarcoma, skin, dog. The plump neoplastic endothelial cells are forming irregular blood spaces and filling the trabeculae in between. HE. 30) Canine hemangiosarcoma, skin, dog. The neoplastic cells show intense immunolabelling. IHC for vascular endothelial growth factor (VEGF). 31) Canine rhabdomyosarcoma, skin, dog. The neoplasm is formed of numerous pleomorphic neoplastic cells with frequent atypical mitosis and few skeletal muscle bundles. HE. 32) Canine epitheliotropic lymphoma, skin, dog. The neoplastic T-cells are infiltrating the dermis with increased affinity for epidermis and adnexal epithelium. HE. 33) Canine epitheliotropic lymphoma, skin, dog. The neoplastic cells show intense immunolabelling. IHC for CD3. 34) Canine mast cell tumor, skin, dog. The neoplastic mast cells are round, monomorphic with abundant basophilic granules. HE. Inset: Canine mast cell tumor, skin, dog. The metachromatic granules of mast cells are dispersed in the cytoplasm. Toluidine blue. 35) Canine cutaneous histiocytoma, skin, dog. The dermis is densely packed by slightly pleomorphic cells with little or no stroma. HE. 36) Canine sebaceous hamartoma, skin, dog. Proliferating mature sebaceous glands are found in the mid dermis with marked peri-glandular fibrous tissue proliferation. HE. 37) Canine transmissible venereal tumor, skin, dog. The neoplasm is formed of uniform round cells with vacuolated cytoplasm as well as frequent mitotic figures and scanty stroma. HE.
Figure 26-37: 26) Canine peripheral nerve sheath tumor, skin, dog. The spindle neoplastic cells are arranged in wavy bundles, palisades and whorls. HE. 27) Canine peripheral nerve sheath tumor, skin, dog. The neoplastic cells show moderate immunolabelling. IHC for glial fibrillary acidic protein (GFAP). 28) Canine hemangioma, skin, dog. The tumor is formed of variably sized blood spaces containing erythrocytes and lined by a single layer of uniform endothelial cells. HE. 29) Canine hemangiosarcoma, skin, dog. The plump neoplastic endothelial cells are forming irregular blood spaces and filling the trabeculae in between. HE. 30) Canine hemangiosarcoma, skin, dog. The neoplastic cells show intense immunolabelling. IHC for vascular endothelial growth factor (VEGF). 31) Canine rhabdomyosarcoma, skin, dog. The neoplasm is formed of numerous pleomorphic neoplastic cells with frequent atypical mitosis and few skeletal muscle bundles. HE. 32) Canine epitheliotropic lymphoma, skin, dog. The neoplastic T-cells are infiltrating the dermis with increased affinity for epidermis and adnexal epithelium. HE. 33) Canine epitheliotropic lymphoma, skin, dog. The neoplastic cells show intense immunolabelling. IHC for CD3. 34) Canine mast cell tumor, skin, dog. The neoplastic mast cells are round, monomorphic with abundant basophilic granules. HE. Inset: Canine mast cell tumor, skin, dog. The metachromatic granules of mast cells are dispersed in the cytoplasm. Toluidine blue. 35) Canine cutaneous histiocytoma, skin, dog. The dermis is densely packed by slightly pleomorphic cells with little or no stroma. HE. 36) Canine sebaceous hamartoma, skin, dog. Proliferating mature sebaceous glands are found in the mid dermis with marked peri-glandular fibrous tissue proliferation. HE. 37) Canine transmissible venereal tumor, skin, dog. The neoplasm is formed of uniform round cells with vacuolated cytoplasm as well as frequent mitotic figures and scanty stroma. HE.
Discussion
The skin is the most common anatomical site for development of neoplasms due to its continuous exposure to risk factors (physical, chemical and environmental [e.g. ultraviolet light]. A wide variety of tumor types can be found in the skin, subcutaneous tissue, and adnexa (Bronden et al., 2010; Dobson et al., 2002; Hueper, 1963). The frequency of various types of skin tumors in a particular geographic region should be recorded to collect information for potential investigations. Awareness of the frequency of various tumor types of skin tumors in a certain population enables clinicians to improve their diagnosis and to evaluate more appropriate treatment plans for their patients (Strafuss, 1985b), and this is the key reason for performing epidemiological studies of this kind (Kaldrymidou et al., 2002). The current study described the epidemiological trends of canine cutaneous tumors in Egypt and provided extensive information on the breed, age, sex, and tumor anatomical site of 118 canine skin biopsy and surgical excision cases submitted to the laboratory over the past 2 years. However, the relatively limited sample size compared to previous studies (Graf et al., 2018; Villamil et al., 2011), this study is useful to elucidate frequently diagnosed tumor types, common dog breeds at risk, and anatomical sites of canine cutaneous tumors in Egypt.
In our study, the percentage of malignant tumors was higher than the percentage of benign tumors (57.63% vs. 42.37%), which is consistent with the previous studies (Kok et al., 2019; Moraes et al., 2009; Souza et al., 2006) The percentage of benign tumors may be underestimated, as cases have mostly been obtained from referral veterinary hospitals. Moreover, benign tumors have not been surgically resected or have not been regularly submitted for histological examination. More than 50% of the tumors obtained from dogs between 5 to 9 years of age could confirm an increased risk of developing canine skin tumors at an older age, as previously described (Graf et al., 2018; Kok et al., 2019). The percentage of tumor samples submitted from male dogs was higher than that of female dogs (52.67% vs 32.14%). These findings are consistent with most previous studies (Bronden et al., 2010; Kaldrymidou et al., 2002; Merlo et al., 2008).
There were differences in the frequency of the five most common skin tumor types reported in this study (basal cell carcinoma, papilloma, infundibular keratinizing acanthoma, liposarcoma, and fibrosarcoma) when compared to previous literatures (Graf et al., 2018; Kok et al., 2019; Moraes et al., 2009). This is undoubtedly due to variations in tumor samples, classification systems, and follow-up diagnostic recommendations. Moreover, the discrepancy in tumor frequency may be related to the population of dog breeds in the studied region and to environmental factors, in particular exposure to ultraviolet light, which is responsible for the development of certain skin tumors (Brodey, 1970; Kaldrymidou et al., 2002; Pakhrin et al., 2007).
Contrary to a previous study (Stockhaus et al., 2001), the present study found that basal cell carcinoma was the most commonly diagnosed skin tumor types. Golden Retriever and German Shepherd were predisposed to basal cell carcinoma in our study, which needs further studies with a larger sample size to validate the hereditary factor in tumor development. In our study, basal cell carcinoma was often found on the head which was consistent with previous reports (Goldschmidt and Goldschmidt, 2016; Goldschmidt and Hendrick, 2002; Strafuss, 1976). Our findings revealed a higher predisposition for basal cell carcinoma in males compared to females, in contrast to a previous report (Goldschmidt and Hendrick, 2002).
The high incidence of cutaneous papilloma in dogs is well-documented (Goldschmidt and Goldschmidt, 2016). The second most common tumor type in the present study was cutaneous papillomatosis. In accordance with a previous report (Goldschmidt and Goldschmidt, 2016), young dogs below the age of 1 up to 5 years were affected. Our findings, in contrast to a previous report (Goldschmidt and Goldschmidt, 2016), suggest a higher predisposition for papilloma in male dogs compared to females. In our study, Baladi breed was predisposed to papillomatosis, although it has been reported that French Bulldog, Rhodesian Ridgeback, Whippet, Vizsla, and Bull Mastiff are at increased risk of developing cutaneous papillomatosis (Goldschmidt and Goldschmidt, 2016).
Being the third most common cutaneous neoplasm, infundibular keratinizing acanthoma was often found on the trunk and increased predisposition in mixed breeds. However, infundibular keratinizing acanthoma was predominantly found in Norwegian Elkhound as described in a previous literature (Stannard and Pulley, 1975). It was previously reported that dogs are the only species affected (Goldschmidt and Goldschmidt, 2016). No sex predilection was observed. In accordance with the literature (Rudolph et al., 1977), the age range of affected dogs in our study was 1-7 years suggesting that infundibular keratinizing acanthoma occurs in young and middle-aged dogs. Infundibular keratinizing acanthoma should be distinguished from well-differentiated squamous cell carcinoma. Infundibular keratinizing acanthoma develops as solitary or multiple cysts filled with keratin (Rudolph et al., 1977). On the other hand, well-differentiated squamous cell carcinoma occurs as an erosive, plaque-like lesion developed from islands and trabeculae of squamous cells into the dermis (Gross et al., 1992). Infundibular keratinizing acanthoma is a benign tumor, thus, microscopic characteristics such as high mitotic index and invasive behavior may also be useful in differentiating infundibular keratinizing acanthoma from well-differentiated squamous cell carcinoma (Aroni et al., 2007; Bongiovanni et al., 2008; Romanucci et al., 2005).
In soft tissue sarcomas, liposarcomas and fibrosarcomas were the fourth and fifth most common encountered malignant neoplasms, respectively, in agreement with previous studies (Dobson et al., 2002; Graf et al., 2018). Liposarcomas were most common in dogs (Hendrick, 2016) and in our study, often found on the trunk. Labrador Retriever was predisposed to liposarcoma and this finding is supported by a previous study (McSporran, 2009). Our findings, in contrast to a previous report (Hendrick, 2016), suggest a higher predisposition for liposarcoma in male dogs compared to females. Fibrosarcomas had a wide age range (2-12 years). No breed or tumor anatomic site predispositions were observed in fibrosarcomas which are in contrast to previous reports (Hendrick, 2016; Kok et al., 2019). Fibrosacromas were predisposed in female dogs than males.
In conclusion, the current study demonstrated the epidemiology of canine cutaneous tumors in Egypt. Basal cell carcinoma, papilloma, infundibular keratinizing acanthoma, liposarcoma, and fibrosarcoma were the most common tumor types. Golden Retriever, German Shepherd, Labrador Retriever, Mixed breed, and Rottweiler were predisposed to the development of cutaneous tumors. The results from this study will help veterinarians to plan their therapeutic approaches to improve the quality of life of their patients.
Acknowledgments
The authors offer their sincere appreciation for cases provided by different veterinary clinics in Egypt; The ARK veterinary clinic (Dr. M. Abdelaziz and Dr. Mina Roshdy), Pawsitive veterinary clinic (Dr. Marco Kelleny), Modern veterinary clinic (Dr. Aziz Sharaf Eldin and Dr. Moataz Shaaban), Country club veterinary clinic (Dr. Hatem Elsherbiny), and Dr. Nevein H. Hammad’s veterinary clinic.
Conflict of Interest
None.
authors contribution
Coceptualization, BH, AA, MM; Sample collection, AS; Diagnosis, MM, AA; Methodology, investigation and data curation, BH, AA, MM; Writing the original draft BH, HA; Review and editing, All authors; statistical analysis, HA. All authors read and approved the final manuscript.
References





