Advances in Animal and Veterinary Sciences
Research Article
Pathological Identification of Egyptian Local Velogenic Newcastle Disease Virus Genotype VIId in Layer Chickens
Mahmoud Fawzy, Manal Afify*, Youssef, I. Youssef
Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University, 12211; Giza, Egypt.
Abstract | Newcastle disease (ND) still threatening the poultry industry in Egypt despite stringent vaccination programs. This study was performed to evaluate the pathological lesions of Newcastle disease virus (NDV) genotype VIId in different organs of commercial layer chickens (ND antibodies-free). Therefore, forty, 1-day-old chicks were divided into 2 equal groups with 20 birds each. Group 1 served as the non-infected (negative control) group, while group 2 was infected at 28 days old by intranasal inoculation of 0.2 ml containing 106 EID50 of NDV genotype VII. Tissue samples from trachea, lung, spleen and liver were collected from dead infected chickens at 4 days post-infection (dpi) and 6 dpi for detection of NDV RNA by real time Polymerase chain reaction (rRT-PCR). Tissue sections from lung, trachea, spleen, liver and proventriculus from the infected group and non-infected group were collected for histopathology at 5 dpi. The clinical signs in infected group appeared at 3 dpi and lasted for the 6th dpi represented by obvious greenish droppings at 5th dpi, leg paralysis and torticollis at the 4th and 5th dpi. Mortality was 10, 6 and 3 birds at 4 dpi, 5 dpi and 6 dpi respectively in infected group with total mortality rate 95%. Grossly, enlarged mottled spleen, severe hemorrhages on the tips of periventricular glands, severe muscle congestion, hemorrhagic ulcers on cecal tonsils and severe congestion with hemorrhages on the thymus gland at 4th and 5th dpi. Detection of NDV nucleic acid by rRT-PCR revealed highest detection rate (80% and 100%) in respiratory tissues (pooled trachea and lung), moderate detection rate (60% and 66.7%) in spleen and lowest detection rate (30% and 33.3%) in liver at 4 dpi and 6 dpi respectively. Histopathological investigation of different organs revealed pneumonia, tracheitis, spleenitis with lymphoid depletion, hepatitis and moderate proventriculitis. We conclude that our velogenic Newcastle disease virus (vNDV) strain is viscerotropic neurotropic pathotype exhibited high morbidity and mortality rates (95%) associated with immunosuppression in infected layer chickens. Detection of NDV RNA by rRT-PCR in our infected chicken organs emphasizing tissue tropism and distribution of vNDV in different organs, also suggesting the use of rRT-PCR in detection of NDV infected organs. further periodical investigations are needed to evaluate vNDV pathogenesis in chickens with considering the virus distribution pattern in different tissues.
Keywords | Experimental infection, Genotype VII, Histopathology, NDV, rRT-PCR
Received | August 01, 2021; Accepted | September 3, 2021; Published | October 15, 2021
*Correspondence | Manal Afify, Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University, 12211; Giza, Egypt; Email: manal.afify@cu.edu.eg
Citation | Fawzy M, Afify M, Youssef, Youssef I (2021). Pathological identification of egyptian local velogenic newcastle disease virus genotype VIId in layer chickens. Adv. Anim. Vet. Sci. 9(12): 2054-2061.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2054.2061
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Fawzy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Newcastle disease virus (NDV) is one of the most harmful poultry pathogens threatening the poultry industry causing a highly contagious septicemic, fatal and destructive disease that attacks mainly chicken and turkeys (Khader et al., 2020). Since its first appearance in Indonesia in1926 (Alexander, 2000) the NDV is still widespread in most countries worldwide with the only exception; The Oceania countries that showed a relative freedom from the disease (Dimitrov et al., 2017). In Egypt, NDV had been firstly identified in 1948 (Daubney and Mansy, 1948). The disease became widely disributed throughout the country and recorded as an endemic disease by the beginning of the 1960 (El-Nassary and Eskarous, 1960). According to their pathogenicity pattern, NDV is classified into velogenic (the most pathogenic), mesogenic (moderate pathogenic) and lentogenic strains (low pathogenic), while based on the tissue tropism and the developed clinical signs, the NDV is classified into viscerotropic, pneumotropic and neurotropic (Aldous and Alexander, 2001). High mortalities with nervous and intestinal pathology are recorded with viscerotropic velogenic and neurotropic phenotypes (Alexander et al., 2012; Nakamura et al., 2008). The main clinical symptoms of NDV are respiratory distress, diarrhea, and circulatory disturbance and in chronic cases, impairment of the central nervous system (Alexander et al., 1992). In chickens, the pathogenicity of NDV vary widely but mainly based on virulence of the virus. Other factors determine the pathogenicity of the disease such as the host species, age, immune status, infection with other organisms as well as dose, the route of administration and environmental stress. In some circumstances, infection with a virulent strain causes sudden deaths without major clinical signs in young chickens, while in adult birds the disease may be more protracted and less sever clinical signs such as respiratory distress including rales, sneezing, and wet eyes and/or nervous manifestations. (Cheville, 1972; Brown et al., 1999). NDV or avian paramyxovirus 1 (APMV-1) is a non-segmented, single-stranded, negative-sense RNA virus in the family Paramyxoviridae (Phillips et al., 1998; de Leeuw and Peeters, 1999). Phylogenetically, based on the genome length and sequence of the F gene, NDV strains have been divided into classes I and class II (fifteen genotypes I-XV). Class I include avirulent strains and comprise a single genotype while class II contains 15 genetic groups including 10 previously established (I–IX, and XI) and five new genotypes (X, XII, XIII, XIV and XV). Class II also contains both virulent and avirulent vaccine strains such as LaSota and Hitchner B1 being used worldwide (Diel et al., 2012; Huang et al., 2004). Currently, the circulating strains from genotypes V, VI, and VII of class II are predominantly associated with disease outbreaks worldwide (Aldous et al., 2003). Genotype VII was initially divided into two sub genotypes: VIIa, representing viruses that emerged in the 1990 s in the Far East and spread to Europe and Asia, while VIIb, representing viruses that emerged in the Far East and spread to South Africa (Aldous et al., 2003). The two sub-genotypes of VII are further divided into VIIc, d, and e, which represent isolates from China, Kazakhstan, and South Africa (Bogoyavlenskiy et al., 2009; Wang et al., 2006) in the same time, VIIf, g, and h, represent African isolates (Snoeck et al., 2009). In Egypt, the sub-genotype VIId is predominant causing several ND outbreaks in poultry (Radwan et al., 2013). NDV causes pathological changes in different organs which include tracheitis, pneumonia, pericarditis, myocarditis, and catarrhal proventriculitis. Catarrhal enteritis, typhlitis, pancreatitis, perihepatitis, and nephritis have also been reported in NDV-infected poultry. Also Splenitis, atrophy of the Bursa of Fabricius, and encephalitis accompanied by lymphocyte and macrophage infiltration were also mentioned (Brar et al., 2017; Etriwati et al., 2017). The present study was designated to investigate the pathogenesis of NDV genotype VIId through recording the clinical signs, morbidity rate, mortality rate and pathological lesions with detection of histopathological alterations and virus distribution in different tissues according to r RT-PCR.
Materials and Methods
Specific pathogen free (SPF) eggs
SPF Embryonated Chicken Eggs (ECE) obtained from Nile SPF (Koom Oshiem, Fayoum, Agriculture Research center- Ministry of Agriculture) was used for passage and titration of the isolated NDV isolate.
Experimental chicks
Forty 1 day- old male layer chicks obtained from commercial poultry company were used for pathogenicity assessment of NDV isolate (NDV/ chicken/FVCU/ Qalubia /Egypt/2019). Chicks were reared in previously cleaned and disinfected separate rooms on deep litter system with straw under optimal conditions from lightening, temperature and humidity. Birds were provided with commercial broiler plated (starter and grower) ration free from medication and provided with clean water ad-libitum. All procedures and bird handling were approved ethically by Institutional Animal Care and Use Committee (IACUC), Cairo University, Egypt (Approval number, CU/II/F/5/21).
Newcastle disease virus (NDV)
Hemagglutination (HA) antigen
ND La Sota vaccinal strain were propagated in ECE and diluted to 4 HAU to be used as HA antigen in Hemagglutination inhibition (HI) testing of ND antibody.
NDV for pathogenicity study
Velogenic Newcastle disease virus (vNDV) local isolate (NDV/ chicken/FVCU/ Qalubia /Egypt/2019) isolated by authors during 2019 from 45-day old chicken flock suffered from respiratory manifestation and mortalities (5.4%) allocated in Qalubia governorate, molecularly characterized and submitted to genbank under accession numbers (MW172503). The used strain is related to NDV Genotype VIId.
Infectivity titration in ECE
The propagated NDV suspension was titrated in [9-10] day old SPF ECE. 10-fold serial dilutions of the virus were prepared in saline solution contains antibiotic. Virus suspensions were inoculated into 5 embryos for each dilution via allantoic sac (0.2 ml per egg). The inoculated embryos were incubated at 37°C and candled twice daily for 6 days. Dead embryos in first day were considered as nonspecific deaths. Slide haemagglutination (HA) test was applied on the allantoic fluid of inoculated chicken embryos to detect the HA-positive eggs. It was carried out according to the standard method described by (Anonymous, 1971; Grimes, 2002) and EID50 was calculated according to Reed and Muench (Reed and Muench, 1938). The inoculation dose was 106 EID50/bird via intranasal instillation.
Experimental design
Forty 1 day- old male layer chicks were reared in disinfected separated rooms; feed and water were provided ad-libitum. Blood samples were randomly collected at one day- old, 14 day- old and 28 day of age (date of infection) from jugular vein of chickens (10 birds) (2 ml for each) on plain test tubes to separate serum to be used in HI test according to (OIE, 2012) using 1% freshly prepared chickens RBCs suspension in order to monitor the level of NDV maternal antibodies to detect the date of infection then chicks were divided into two groups (20 per each) as follow; control non infected group (group 1), infected group at 28 days old (group 2) were infected by intranasal route with 0.2ml of 106 EID50 of NDV/ bird. Infected birds were observed daily for 10 days post infection (dpi) with recording of morbidity and mortality rates, clinical signs and pathological lesions (either macro or micro examination). Tissue samples from trachea, lung, liver and spleen were collected from dead infected bird to detect viral RNA in these tissues by rRT-PCR. Tissue sections from trachea, lung, liver, proventriculus and spleen were collected from all experimental groups at the 5th dpi for histopathological examination.
Nucleic acid (RNA) Extraction and rRT-PCR for detection of virus nucleic acid in different tissues
The viral RNA extraction was applied individually on the collected samples; spleen, liver and a pool of trachea and lung by Easy PureR Viral DNA/ RNA Kit (transbionovo, China) in accordance with manufacturer’s instructions. Single step rRT-PCR assays using Trans ScriptR Probe One- Step qRT- PCR SuperMix (transbionovo, China) were conducted using specific oligonucleotide primers and probes for vNDV (Wise et al., 2004). The final reaction volume was 20 μL including 4 μL RNA template, 10 μL 2X TransStartR Probe qPCR mix, 0.4 μL Trans ScriptR one- step RT/RI enzyme mix, 0.4 μL of each of the forward and reverse primers, and 0.4 μL probe together with 4.4 μL nuclease free water. Thermocycling rRT-PCR conditions were 45oC for 5 min, 94oC for 30 sec, followed by 40 cycles at 94 oC for 5 sec and 30 sec at 54oC and extension at 72°C for 10 sec.
Histopathological examination
Tissue sections from lung, trachea, spleen, liver and proventriculus were collected from the infected group and non-infected group and fixed on formalin 10%. The specimens were routinely processed in paraffin embedding method, sectioned, and stained with Hematoxylin and Eosin (H & E) for light microscopic examination according to Bancroft and Gamble (Bancroft and Gamble, 2008).
Results and Discussion
Monitoring of NDV specific antibody titers
NDV mean antibody titers of sera collected at 1 day old, 14 days old and 28-day old chickens to detect the date of infection were 6.00 ± 0.63, 3.30 ± 0.45 and 0.00 ± 0.00 respectively. At 28 days of age, birds were found to be free from any detectable antibody (sero negative chickens). (Values are expressed as mean of Log 2±SE).
Clinical signs and gross pathology
Clinical signs begin at the 3rd dpi with marked depression with sleepy appearance, ruffled feather, greenish lose dropping and decrease in feed and water intake with no mortality. In 4th dpi: severe depression with sleepy appearance, foamy conjunctivitis, periorbital edema Figure 1A, neurological signs (leg paralysis) Figure 1B, marked decrease in feed and water intake and severe greenish lose dropping with sudden high mortalities Figure 1C. In the 5th dpi: obvious severe greenish lose dropping Figure 1D, neurological signs (torticollis) Figure 1E with severe loss of appetite and mortalities. 19 out of 20 birds in infected group died during 4th to 6th dpi Figure 2 while one bird in the infected group and all birds in the non-infected group remain healthy without obvious clinical signs and mortalities all over the observation period (10 dpi). Therefore, the morbidity rate was 19/20 (95%) also mortalities reach 95% in the infected group. The most prominent gross lesions of dead infected chickens observed during 4th and 5th dpi were enlarged mottled spleen Figure 3A, severe hemorrhages on the tips of periventricular glands Figure 3C, severe muscle congestion Figure 3B, hemorrhagic ulcers on cecal tonsils Figure 3D and severe congestion with hemorrhages on the thymus gland Figure 3E observed on postmortem examination.
Detection of viral nucleic acid (RNA) in different tissues at different intervals post infection (dpi) using rRT-PCR
Viral RNA detected by rRT-PCR in different tissues collected from dead infected birds at 4th and 6th dpi, the result revealed that (8/10) and (3/3) of the pooled trachea and lung tissue samples were positive at the 4th and 6th dpi, respectively, (6/10) and (2/3) of spleen tissue samples were positive at the 4th and 6th dpi, respectively and (3/10) and (1/3) of liver tissue samples were positive at the 4th and 6th dpi, respectively Table 1.

Figure 1: Clinical signs observed in the infected group at 4th and 5th days post- infection (dpi). A, foamy conjunctivitis and periorbital edema were observed at 4th dpi; B, neurological signs (leg paralysis) were observed at 4th dpi; C, sudden high mortalities were observed at 4th dpi; D, greenish lose droppings were observed at 5th dpi; E, neurological signs (torticollis) were observed at 5th dpi.
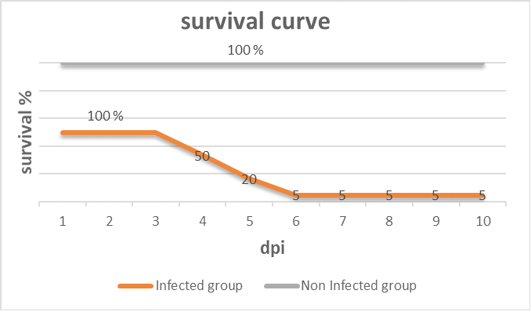
Figure 2: Survival curve after inoculation of 28 days old chickens with vNDV (infected group) and non-infected group of the experiment at different days post- infection (dpi).
Table 1: Detection of ND Viral RNA in different tissues at different days post infection (dpi).
|
Tissue/ organ |
dpi |
Total number of dead birds |
Positive |
|
|
Number |
% |
|||
|
Trachea and lung (pool) |
4 dpi |
10 |
8 |
80 |
|
Liver |
10 |
3 |
30 |
|
|
Spleen |
10 |
6 |
60 |
|
|
Trachea and lung (pool) |
6 dpi |
3 |
3 |
100 |
|
Liver |
3 |
1 |
33.3 |
|
|
Spleen |
3 |
2 |
66.7 |
|
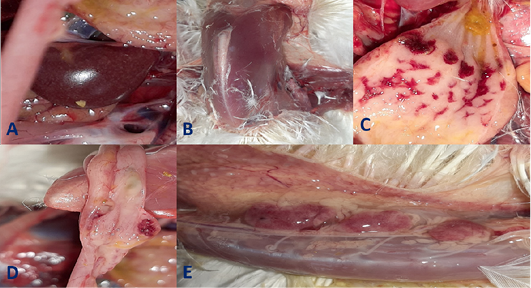
Figure 3: Gross pathological lesions observed in the infected group at 4th and 5th days post- infection (dpi) up on postmortem examination. A, Enlarged mottled spleen; B, severe muscle congestion; C, severe hemorrhages on the tips of periventricular glands; D, hemorrhagic ulcers on cecal tonsils; E, severe congestion with hemorrhages on the thymus gland.
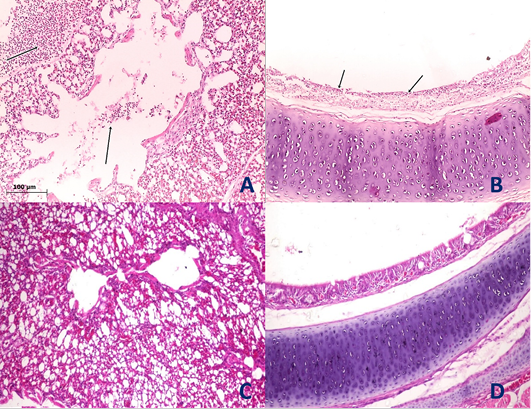
Figure 4: Histological section of lung and trachea of the infected and non-infected group at 5th day post infection (dpi). A, lung showed moderate pneumonia with inflammatory exudate inside tertiary bronchi and secondary bronchi (arrow) with necrosed tertiary bronchi lining; B, trachea revealed tracheitis with noticeable necrosis of lining epithelium and mononuclear cells infiltration (arrow); C, normal lung (non-infected group) with no histopathological alterations; D, normal trachea (non-infected group) with no histopathological alterations.
Histopathological examination
Histopathological examination of the organs including lung, trachea, spleen, liver and proventriculus collected from the infected group and non-infected group at 5th dpi reveals that the lung showed moderate pneumonia with inflammatory exudate inside tertiary bronchi and secondary bronchi (arrow) with necrosed tertiary bronchi lining Figure 4A, trachea revealed tracheitis with noticeable necrosis of lining epithelium and mononuclear cells infiltration (arrow) Figure 4B, spleen showed spleenitis with marked focal necrosis of lymphoid tissue (arrow) Figure 5A, liver showed hepatitis with mononuclear cells infiltration (arrow) and dilatation of hepatic sinusoids (yellow arrow) Figure 5B and Proventriculus suffered from moderate proventriculitis with mononuclear inflammatory cells infiltration within tunica muscularis (arrow) and epithelial cells degeneration Figure 5C. Non infected group showed no histopathological alterations in any examined tissue as illustrated in Figures 4C, 4D, 5D, 5E and 5F.
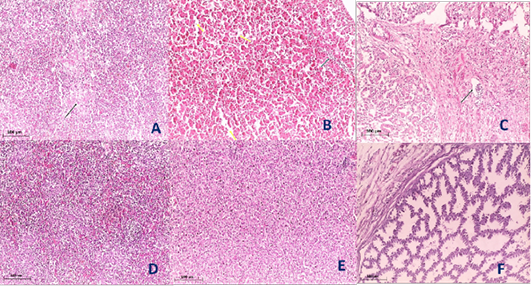
Figure 5: Histological section of spleen, liver and Proventriculus of the infected and non-infected group at 5th day post infection (dpi). A, spleen showed spleenitis with marked focal necrosis of lymphoid tissue (arrow); B, liver showed hepatitis with mononuclear cells infiltration (arrow) and dilatation of hepatic sinusoids (yellow arrow); C, Proventriculus suffered from moderate proventriculitis with mononuclear inflammatory cells infiltration within tunica muscularis (arrow) and epithelial cells degeneration; D, normal spleen (non-infected group) with no histopathological alterations; E, normal liver (non-infected group) with no histopathological alterations; F, normal Proventriculus (non-infected group) with no histopathological alterations.
During the last few years, severe NDV genotype VII outbreaks occurred in poultry flocks in Egypt causing severe economic losses in spite of stringent vaccination programs (Ewies et al., 2017).
Our experimental study is designated to evaluate the pathogenicity of a locally isolated vNDV strain (NDV/ chicken/FVCU/ Qalubia /Egypt/2019) (accession numbers MW172503) in commercial layer chickens. Chickens were infected via intranasal inoculation simulating the natural route of infection under field conditions (Alexander et al., 2012).
Our results revealed high morbidity and mortality rates (95%) in infected chickens indicating that our strain is velogenic, also molecular pathotyping of this strain through partial sequence of F gene including cleavage site motif showed that this strain is velogenic (unpublished data). Otherwise, two experimental studies were recorded using different NDV strains of the same genotype (VIId) in boiler chickens, where one study recorded 72% mortalities at 25 days of age (Khader et al., 2020) and the other study recorded 80% mortalities at 28 days of age (Amer et al., 2018). All of these emphasizing the high morbidity and mortality rates associated with vNDV infection in chickens (Susta et al., 2011).
Notably, the clinical signs appeared at 3 dpi and lasted for the 6th dpi, which is likely attributed to the incubation period of ND (Miller and Koch, 2013). The clinical findings represented by obvious greenish droppings at 5th dpi, leg paralysis and torticollis at the 4th and 5th dpi indicate that our NDV isolate is a viscerotropic neurotropic strain. Similar findings were reported using different NDV strain of the same genotype in broilers (Khader et al.,2020; Mohammed et al., 2019; Mousa et al., 2019) and commercial male layer chickens vaccinated with Hitchner B1 and Lasota live ND vaccines (Amer, 2018). Also, the marked gross and histopathological lesions found in proventriculus confirm the viscerotropic tropism of our NDV strain. Similar findings were reported using different NDV strain of the same genotype in broilers (Mahmoud et al., 2019).
Although the used strains belong to the same genotype, the mortality rate in our infected layer chickens (95%) was higher than that recorded in boilers (Khader et al.,2020; Amer et al., 2018), also the onset of nervous signs was earlier in our infected layer chickens (4 dpi) compared to that recorded in boilers (Mohammed et al., 2019; Mousa et al., 2019), suggesting that layers possibly more vulnerable to velogenic neurotropic NDV infection (King, 1996). However, molecular fingerprinting is another factor to be investigated.
The necropsy of our infected chickens revealed severe systemic affections of muscles and internal organs like proventriculus, intestine and spleen emphasizing that our NDV strain is velogenic and able to replicate systemically in different organs (Eze et al., 2014). Similar findings were reported using different NDV strain of the same genotype in broilers (Mousa et al., 2019).
The marked gross affections in the gut associated lymphoid tissues including cecal tonsils and proventriculus gland as well as immune organs like spleen and thymus gland highlight the immunosuppressive effect of vNDV (Ezema et al., 2016). Similar findings were reported using different NDV strain of the same genotype in 30 day- old broiler chickens (Mousa et al., 2019).
detection of NDV RNA by rRT-PCR in spleen in our study suggesting that the gross and histopathological lesions of spleen are attributed to an NDV infection, emphasizing the immunosuppressive effect of NDV (Ezema et al., 2016). Although the detection method was different, (Mousa et al., 2019) recorded similar detection rate (marked expression of viral antigen) in spleen of 30 day- old broiler chickens infected with different NDV strain of the same genotype by immunohistochemistry (IHC), emphasizing that rRT-PCR and IHC could be used for detection of NDV in infected organ (Hoffmann et al., 2009; Naglaa et al., 2014; Hussein et al., 2019).
Although mild respiratory signs, gross and histopathological lesions were recorded in our experiment, the highest detection rate of viral RNA was showed in respiratory organs (lung and trachea) at 4th and 6th dpi, this is basically because all NDVs initially replicate in respiratory system regardless this is because NDV replicates mainly at the portal of entry before it undergoes viraemia and disseminates into visceral organs (Wambura et al., 2006).
Although the detection method was different, (Khader et al., 2020) recorded similar detection rate in trachea and lung (highest viral density in trachea and lung at 5th, 6th and 7th dpi) of 25 day- old broiler chickens infected with different NDV strain of the same genotype by immunohistochemistry (IHC), also (Khader et al., 2020) recorded high viral RNA load in the trachea at 5th dpi by rRT-PCR. All of these emphasizing that rRT-PCR and IHC could be used for detection of NDV in infected organs (Hoffmann et al., 2009; Naglaa et al., 2014; Hussein et al., 2019).
Detection of NDV RNA by rRT-PCR at the 4th and 6th dpi and histopathological changes in liver of our infected chickens possibly attributed to an vNDV infection (Hussein et al., 2019). Similar histopathological findings were reported using different NDV strain of the same genotype in broilers (Aziz-ul-Rahman et al., 2019). Although the detection method was different, (Adi et al., 2012) reported similar detection rate (low viral density) in liver of chickens experimentally infected with viscerotropic vNDV using IHC method emphasizing that rRT-PCR and IHC could be used for detection of NDV in infected organ (Hoffmann et al., 2009; Naglaa et al., 2014; Hussein et al., 2019).
Conclusions and Recommendations
We conclude that our vNDV strain is viscerotropic neurotropic pathotype exhibited high morbidity and mortality rates (95%), gross and histopathological lesions in immune organs and gut associated lymphoid tissues causing immunosuppression in infected layer chickens. Also, detection of NDV nucleic acid by rRT-PCR in our infected chicken organs was higher in respiratory tissues, moderate in spleen and lower in liver emphasizing tissue tropism and distribution of vNDV, also suggesting the use of rRT-PCR in detection of NDV infected organs. further periodical investigations are needed to evaluate vNDV pathogenesis in chickens with considering the virus distribution pattern in different tissues.
Novelty Statement
This manuscript provides a data about clinico pathological picture and virus distribution in different tissues of infected layer chicken using new isolate of NDV related to genotype VII that is contributed in occurrence of severe outbreaks in poultry farms in Egypt.
Author’s Contribution
All authors contributed in practical part (NDV isolation and infectivity titration, evaluation of experimental infection in layer chicken with NDV isolate. Also, all authors contributed in scientific writing and revision of this manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References





