Advances in Animal and Veterinary Sciences
Research Article
Environmental Conditions Associated with Parasitic Infections in Laboratory Mice
Raslan Ain-Fatin1, Saulol Hamid Nur-Fazila1*, Md Isa Nur-Mahiza1, Abd Rahaman Yasmin2, Yong Meng Goh3, Firdaus Mohd-Qawiem1
1Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; 2Department of Veterinary Laboratory Diagnosis, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; 3Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
Abstract | A high parasitic burden in the laboratory mice (Mus musculus) disrupts significant research outcomes. Many factors in the animal facility contribute to parasites manifestation. Presently, the environmental variables on parasites presence in laboratory mice remain scarce in Malaysia. Hence, this study aimed to determine the association of varied environmental conditions towards parasitic infections in laboratory mice. Fifty-four (54) male BALB/c mice were chosen randomly from an animal facility located in Klang Valley, Malaysia. They were placed in three groups of different environmental conditions with 3 replicates for each group. Endoparasites were identified using direct faecal smear, perianal tape test, faecal floatation and gastrointestinal examination techniques. Ectoparasites were detected by fur pluck, tape impression and carcass immersion methods while blood parasites were recognised using blood smear techniques. Samples were taken weekly for a total of 5 weeks. Identification of the parasites was made based on observation and classification of their distinct characteristics under a light microscope. The results revealed that the mice were infected with pinworms; Syphacia obvelata and Aspiculuris tetraptera, but blood parasites were absent. Mites were only detected in the non-regulated environment at a prevalence of 20.4% by the tape impression method. It was contributed by the stressful conditions of the non-regulated environment. There was an association between parasitic levels with various environmental conditions using one-way ANOVA (P < 0.05). Overall, the results varied according to the parasitological methods used, and environmental conditions play roles towards parasites manifestation in the laboratory mice housed in the conventionally maintained animal facility.
Keywords | Environmental conditions, Laboratory mice, Mites, Parasites, Pinworms
Received | August 12, 2021; Accepted | August 18, 2021; Published | October 15, 2021
*Correspondence | Saulol Hamid Nur-Fazila, Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; Email: nurfazila@upm.edu.my
Citation | Ain-Fatin R, Nur-Fazila SH, Nur-Mahiza MI, Yasmin AR, Goh YM, Mohd-Qawiem F (2021). Environmental conditions associated with parasitic infections in laboratory mice. Adv. Anim. Vet. Sci. 9(12): 2047-2053.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2047.2053
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Nur Fazila et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The genetic, biological and well-adaptive behaviour characteristics of the laboratory mouse (Mus musculus) that closely resemble those of humans are contributing factors to be chosen in research. The laboratory animal facility should provide a proper animal housing with adequate conditions and appropriate management to suit the species or strains of the animals maintained. The facility should accommodate the animals’ physical, physiological, and behavioural needs that allow animals to mature and breed while maintaining their health and well-being (National Research Council, 2011). Substantial findings of research involving the experimental animal’s biological processes are influenced by environmental factors. The control of environment variables in the facility plays a vital role when considering animals used in research. Different environmental conditions of animal facilities influence the types of parasites, with evidence of uncommon ones (Ain-Fatin et al., 2020a). The environmental variables in an animal facility involve (i) physical factors such as temperature, relative humidity, lighting, and sound, (ii) psychosocial factors such as animal handling and group composition, (iii) chemical factors such as chemicals used in the facility, and (iv) microbial factors such disease-causing microorganism like viruses, bacteria, fungus and parasites (Huerkamp et al., 2018). These variables are imperative when designing a facility and the operational protocols.
The environmental fluctuations change the animal’s behaviour, physiology and morphology that disrupt the research outcomes (Gordon, 1993). The thermoneutral zone (TNZ) of the mice ranged between 26°C and 34°C. Meanwhile, the recommendation for relative humidity is between 30 to 70% because increased levels contribute to the high ammonia concentrations within the cage (Corning & Lipman, 1991; Hasenau et al., 1993) leads to respiratory irritation and alteration of biological responses (Wang et al., 1998). Air quality has to be maintained by providing frequent ventilation to allow adequate oxygen supply. The air changes per animal room should range at 10 to 15 fresh air and sufficient lighting is provided to ensure enough illumination to animals (National Research Council, 2011) for neuroendocrine regulation of diurnal and circadian cycles (Corning & Lipman, 1991). The fluctuations in environmental factors become stressors that should be excluded from the study. These stressors enhance the risk factors of animals being infected that further interfere with the experimentation (Pam et al., 2013).
Some parasites have been recorded in laboratory rodents (Medeiros, 2012). The common helminths found like Syphacia spp. and Aspicularis spp. are spread by direct ingestion of embryonated eggs found in the faeces (Perec-Matysiak et al., 2006). Clinical signs are unusual unless heavy parasitic levels include pruritus at the perianal region, impaction, or rectal prolapse (Medeiros, 2012). The common ectoparasites found are the fur mites; Myocoptes musculinis (M. musculinis) and Myobia musculi (M. musculi) that transmitted by direct contact with the infected host. Clinical symptoms include pruritus, alopecia, scabbing, and irritation (Baker, 2007). Blood parasites are rarely reported in laboratory rodents, however the mentionable blood parasites include the Plasmodium spp., Hepatozoon spp., and Haemabartonella spp. (Sirois, 2005) and Trypanosoma spp. (Baker, 2007; Pritchett, 2007).
Zoonotic infections were also reported in rodents of laboratory settlings (Bleich, 2008). Parasitized animals can also complicate research by inducing physiological, behavioural, haematological, biochemical, immunological, and pathological changes in the hosts. Parasites trigger the host’s susceptibility to experimental stress, induce tissue damage, stimulate abnormal tissue growth, compete with the host for nutrients, disturb digestibility, reduce the host’s blood volume and body fluids that ultimately affects the research outcomes (Aboel-Hadid and Gamal 2007; Dolatkhah et al., 2017).
Researchers must obtain laboratory animals from a trustworthy source to ensure the reliability and validation of the research findings. From this, early preventative measures should be considered before choosing animals for research studies. Due to limited studies conducted on the environmental factors on parasitic infection, we aim to assess the parasites present in various environmental conditions of the conventionally maintained animal facility.
MATERIALS & METHODS
Ethical approval
All protocols described were undertaken following criteria approved by the Universiti Putra Malaysia (UPM) Institutional Animal Care and Use Committee (IACUC) with an approval code of UPM/IACUC/AUP-R087/2018.
Experimental animals
Fifty-four (54) adult male BALB/c aged 4 to 5 weeks were chosen randomly from a conventionally maintained animal house located in Klang Valley, Malaysia. They were divided into three groups placed in individually ventilated cages in a regulated environment (Group A), open-top cages in a regulated environment (Group B) and open-top cages in a non-regulated environment (Group C). All mice provided with rodent chow twice daily and unrestricted filtered water through drinking bottles.
The mice placed in the regulated environment were maintained in an indoor conventional facility either in individually-ventilated cages (IVC) for Group A and open-top cages (OTC) for Group B. Cages and bedding were changed twice-weekly in a dedicated biosafety cabinet. The room was maintained on a 12 hours light: dark cycle, the temperature of 18 ± 2 °C by air-conditioning, and relative humidity of 30% to 50%. In Group C, they were maintained in the non-regulated environment that was placed in OTC with wood-shavings bedding. Cages and bedding were changed twice-weekly in the same room without a biosafety cabinet. The room was maintained on a 12 hours light : dark cycle with LED lights switched on and off, however, the room was also exposed to daylight from windows in the room. The room could be influenced by the fluctuations in the environment as a ceiling fan was used with unregulated temperature, relative humidity, and ventilation.
Detection of parasitic infection
The parasitological methods were conducted for all 54 animals within a 5-week time frame following studies by Parkinson et al. (2011) and Gerwin et al. (2017). Detection of endoparasites was performed using perianal tape test, direct faecal smear, faecal floatation, and direct examination of gastrointestinal tract contents. Ectoparasite detection was conducted using tape impression, fur pluck and carcass immersion. Examinations of blood parasites were carried out using thin and thick blood smear techniques. The morphology of the parasites was identified using a compound microscope at magnifications of 10× and 40× objectives.
Statistical analysis
Statistical analysis was carried out using the software Statistical Packages for the Social Sciences (SPSS). Multivariate analysis of variance (MANOVA) of repeated measures was used for parasitological methods bound towards the 5-weeks sampling to assess parasitic infection of different environmental conditions in BALB/c mice and it is considered as significant when P < 0.05. One-way analysis of variance (ANOVA) was used for parasitological methods performed once to assess the level of the parasitic infection of different environmental conditions and it is considered as significant when P < 0.05.
RESULTS
The effect of environmental conditions on parasitic infection in laboratory mice was identified using various parasitological methods. Comparisons were made between three groups; Group A (individually-ventilated cages in a regulated environment), Group B (open-top cages in a regulated environment), and Group C (open-top cages in the non-regulated environment).
Helminths of S. obvelata and A. tetraptera were found in the laboratory mice when subjected to the direct faecal smear method. Identification was made based on distinctive characteristics of anterior and posterior anatomical structure with additional ova morphology. The helminths are differentiated by morphological differences between the ova and adult worms (Baker, 2007; Pritchett, 2007). Perianal tape test revealed the presence of S. obvelata ova that is recognized as a pointed oval with a flattened side at the measurement of 134 x 36 µm (Pritchett, 2007). The A. tetraptera ova were identified by faecal floatation with the characteristics of ellipsoidal and symmetrical in shape at the measurement of 86 x 37 µm (Pritchett, 2007).
The association between parasitic infection and different environmental conditions were demonstrated in Table 1. Using the direct faecal smear and perianal tape methods, although various environmental conditions had no difference to endoparasite infection, an association was seen at different time points towards endoparasite infection (P = 0.000), as tested by the within-subject effect. Similarly, although no difference was obtained by the faecal floatation method, an association was observed between different time points (P = 0.002).
Direct examination of gastrointestinal contents revealed gravid S. obvelata female worms with an absence of A. tetraptera. The amount of adult female worms found for each group was summarised in Figure 1. Findings revealed the group placed in the non-regulated environment had the most number of adult worms found with a total of 53 worms but Group B which was placed in the open-top cages in a regulated environment had the most positively infected mice at 100%. However, this method showed environmental conditions were not related to endoparasite infection.
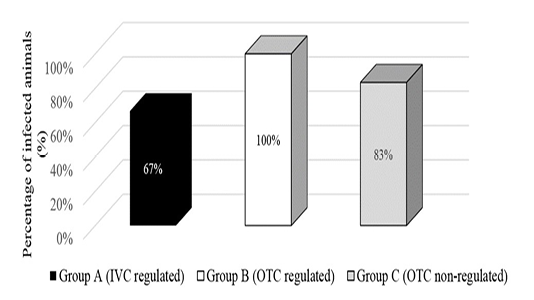
Figure 1: Presence of Syphacia oblevata worms by direct examination of gastrointestinal contents in different environmental condition groups.
The ectoparasites recognized are alleged to be known as the arachnid; mite as observed in Figure 2, based on their shape, limbs, absence of wings and antennae on the parasite (Hoy, 2011). However, it is speculated that the mite belongs to the family of Psoroptidae due to its oval appearance, long and pointed chelicerae, and triangular gnathosoma (Baker, 2007). Majority of the mites found on the tape impression test were at the nymph stage due to their appearance with three pairs of limbs (Wall & Shearer, 1997).
From the fur pluck method, mites were observed in two (2) out of 54 mice (3.7%) in Group C that were placed in a non-regulated environment. However, mice placed in the regulated environment were free from ectoparasites throu-
Table 1: Relationship between environmental conditions and endoparasitic levels in BALB/c mice using various parasitological methods by repeated measures ANOVA method.
| Source | Test | Sum of square (ss) | df | MS | F |
P-value (Sig.) |
| Direct faecal smear | ||||||
| Between-subject effects Group | 0.178 | 2 | 0.089 | 0.286 |
0.761 |
|
| Within-subject effects | ||||||
| Time | Sphericity Assumed | 0.756 | 4 | 0.189 | 0.739 |
0.575 |
| Time x group | Sphericity Assumed | 0.711 | 8 | 0.089 | 0.348 |
0.938 |
| Perianal tape test | ||||||
| Between-subject effects Group | 0.924 | 2 | 0.462 | 0.588 | 0.559 | |
| Within-subject effects | ||||||
| Time | Sphericity assumed | 1.345 | 4 | 0.336 | 5.999 | ***0.000 |
| Greenhouse-Geisser | 1.345 | 3.269 | 0.412 | 5.999 | ***0.000 | |
| Huynh-Feldt | 1.345 | 3.665 | 0.367 | 5.999 | ***0.000 | |
| Time x group | Sphericity Assumed | 1.065 | 8 | 0.133 | 2.374 | *0.018 |
| Greenhouse-Geisser | 1.065 | 6.538 | 0.163 | 2.374 | *0.028 | |
| Huynh-Feldt | 1.065 | 7.329 | 0.145 | 2.374 | *0.022 | |
| Faecal floatation | ||||||
| Between-subject effect Group | 0.711 | 2 | 0.356 | 1.455 |
0.305 |
|
| Within-subject effects | ||||||
| Time | Sphericity Assumed | 3.022 | 4 | 0.756 | 5.667 |
***0.002 |
| Greenhouse-Geisser | 3.022 | 2.441 | 1.238 | 5.667 |
*0.012 |
|
| Huynh-Feldt | 3.022 | 4.000 | 0.756 | 5.667 |
***0.002 |
|
| Time x group | Sphericity Assumed | 2.178 | 8 | 0.272 | 2.042 |
0.084 |
***P<0.005,**P<0.01,*P<0.05: considered to be significantly different. df, degree of freedom; MS, mean square; F, ratio of two variances.
Table 2: Relationship between environmental conditions and ectoparasitic levels in BALB/c mice using various parasitological methods by one-way ANOVA method.
| Source | Test | Sum of square (ss) | df | MS | F |
P-value (Sig.) |
| Fur pluck | ||||||
| Between-groups | 0.148 | 2 | 0.074 | 2.125 | 0.130 | |
| Within-groups | 1.778 | 51 | 0.035 | |||
| Total | 1.926 | 53 | ||||
| Tape impression | ||||||
| Between-groups | 4.481 | 2 | 2.241 | 26.714 |
***0.000 |
|
| Within-groups | 4.278 | 51 |
0.084 |
|||
| Total | 8.759 |
53 |
||||
***P<0.005: considered to be significantly different. df, degree of freedom; MS, mean square; F, ratio of two variances.
-ghout the 5-weeks. Under the tape impression test, mites were detected in 11 out of 54 mice (20.4%) in Group C, but the complete absence of mites was placed in the regulated environment of Group A and B. There was a significant difference between environmental conditions towards ectoparasite infestation by tape impression method (P = 0.001, Table 2). Nevertheless, ectoparasites were not detected using the carcass immersion method at the end of the 5-weeks. Meanwhile, none of the blood parasites was observed in any of the samples throughout the research.
Based on the Duncan Multiple Range Test illustrated in Figure 3, Group A (2.0) and Group B (2.0) showed significant differences with Group C (1.4) at a 5 percent level on the number of infected mice with ectoparasites.
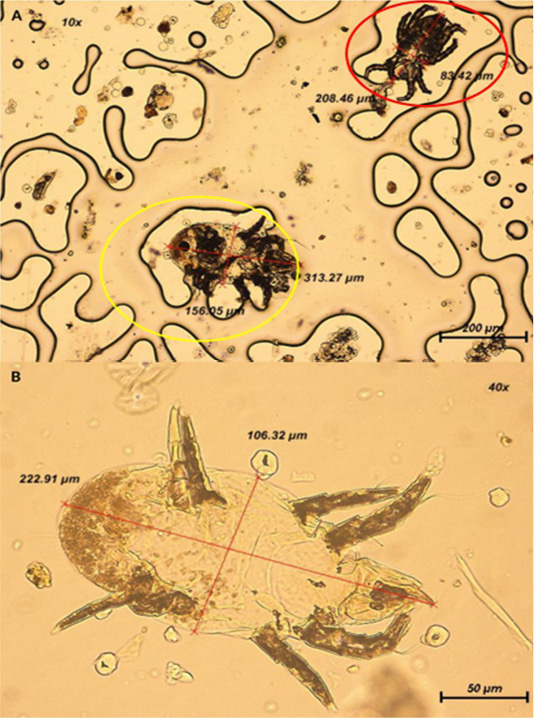
Figure 2: (A) Presence of two mites at 100x magnification. Yellow circle: Mite is oval-shaped with shorter III and IV limbs; Red circle: Mite is elongated in shape. (B) Closer view of mite with appearance of 4 pairs of segmented limbs at 400x magnification.
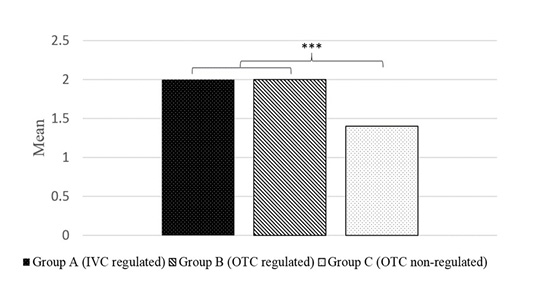
Figure 3: Post-hoc test on relationship between environmental conditions and parasites present in BALB/c mice by gastrointestinal examination method. Values in Group A and B differed significantly than Group C at P < 0.005.
DISCUSSION
This study revealed the presence of helminths belong to the family of Oxyuridae; Syphacia obvelata and Aspiculuris tetraptera which are opportunistic pathogens that are commonly pinworms found in animal facilities (Pinto et al., 2001). Only one out of 13 laboratory mice colonies in animal facilities in Brazil was found to be negative for pinworms (Bicalho et al., 2007). Although non-pathogenic, pinworms compromise the host’s growth, haemopoiesis (Burgarski et al., 2006), nutrient digestibility (Plachỳ et al., 2016), and intestinal electrolyte transportation (Philpott et al., 1992). Parasitized animals are commonly subclinical, and affect their physiology, immunology, and biochemical parameters which influence the experimental model (Bicalho et al., 2007). Pinworms influence nonrelated laboratory experiments by modulating immune responses by eliciting the proliferation of T and B lymphocytes in the spleen and lymph nodes (Michels et al., 2006). Therefore, the parasites disturb the function of the gastrointestinal tract and alter the host immune responses that influence the animal studies primarily related to digestive and immunological model. Thus, the animals infected by pinworms are also not suitable for growth and behavioral studies.
The current study shows that varied environmental conditions bring an impact on the ectoparasitic infestation, but less likely to affect endoparasitic infection in laboratory mice. Animal facilities with variable management in the same campus had varying health statuses (Carty, 2008). Previously, the environmental factors that influenced an animal’s responses include temperature, relative humidity, light intensity, noise control, and gaseous contamination (National Research Council, 2011). Stress responses contributed by lighting, noise, cage cleaning, and in-house transportation could influence the animals’ behaviour and metabolism as mentioned by Castelhano-Carlos & Baumans, (2009). Nevertheless, we cannot rule out the factor of the mice’s ability to adjust well towards new environmental changes. A 5-week period of study provides sufficient time for the animals to adapt by minimizing the stress over time as reported recently (Ain-Fatin et al., 2020b). In contrast, a stark difference could be observed in the ectoparasite infestation in various environmental conditions.
Ectoparasite was identified in Group C placed in the non-regulated environment at a prevalence of 20.4% by tape impression test. They were transmitted to humans as pruritus was experienced after handling these animals. Thus, the mites were not species-specific and could transmit between mammals. There is the possibility of wild rodents as the causal due to the proximity of the room to the outside environment compared to the mice placed in the regulated condition equipped with proper biosecurity and double-door systems to prevent the entry of unwanted pests. The mice placed in the non-regulated environment were also susceptible to ectoparasites due to stressful episodes. On an important note, one mouse died on Week 1 of the experiment during handling and the rest appeared to be distressed condition. It has been studied previously that chronic stressors and persistent high glucocorticoid levels can lead to general immunosuppression (Boonstra, 2013) and suppression of skin immune function (Dhabhar & McEwen, 1991). Furthermore, increased glucocorticoid level may help with the continued existence of the ectoparasites on the host due to their immunosuppressive effect (Juliana et al., 2014). Nevertheless, prevention of exposure to fluctuations in the environment would greatly benefit the laboratory animals and become the basis of animal facility design.
The parasitic burden can alter the host’s biology, behaviour and physiology that may compromise the validity of research results. Therefore, it is recommended to continue monitoring the animal’s health status by implementing a quarantine program in the animal facility and effective barrier systems with optimal environmental conditions to keep the animal’s health and well-being under controlled sanitary conditions. Laboratory animal technology such as housing management and sanitary control is imperative to provide better health conditions for the research animals. Personnel working with laboratory animals need to be aware of the risk of parasitic infection. The importance of frequent cleaning and proper ventilation systems can reduce parasitic infection (Najafi et al., 2015). Management of the animal facility should be an important factor for researchers to consider before choosing laboratory animals. Unmonitored animals in the animal facility harbour various underlying diseases that may alter the outcome of research studies. Maintain parasite-free conditions when using experimental murine models for research investigation is needed. Hence, it is vital to ensure the quality and reliability of the source when obtaining laboratory mice for research.
Overall, the study discovered that results vary according to parasitological methods used. The varying environmental conditions influence parasite infection. Nevertheless, we cannot rule out the multiple possibilities of individual age-related resistance that may reduce parasitic infection with age or time (Shibahara, 2000) and their ability to adapt well towards new changes in the environment. On the other hand, it differs for ectoparasites that were found only in the group placed in the non-regulated environment that was significantly associated with the environmental condition by the tape impression test.
ACKNOWLEDGEMENTS
The study was supported by a research grant (UPM/FPV/2020/6300899). The authors wish to thank the staff of Animal Resource Unit and Veterinary Parasitology Laboratory, Faculty of Veterinary Medicine, UPM who helped in the project particularly Mrs. Maizatul, Mr. Rashid, Mr. Zainuddin, Mr. Aizat, and Mr. Ismail.
CONFLICT OF INTERESTS
The authors declared that there is no conflict of interest.
AUTHORS CONTRIBUTION
SHNF designed the study, and RAF conducted the experiment, data analysis and drafted the manuscript. YMG was involved in data interpretation. SHNF, MINM, ABY and FMQ critically revised the manuscript. All authors have read and approved the final manuscript.
REFERENCES





