Advances in Animal and Veterinary Sciences
Research Article
Virulence Potential of Listeria monocytogenes Recovered from Ice cream and Aborted Women Samples in Sohag city, Egypt
Alshimaa A. Hassanien1*, Eman M Shaker2
1Department of Zoonoses, Faculty of Veterinary Medicine, Sohag University, Egypt; 2Department of Milk Hygiene, Faculty of Veterinary Medicine, Sohag University, Egypt.
Abstract | The circulation of virulence strains of L. monocytogenes in food products increased the burden of transmission of these pathogens to human especially those with impaired immunity such as pregnant women leading to abortion. This study detected the existence of L. monocytogenes and some of its virulence genes in ice cream sold in different food premises in Sohag city and aborted women admitted to Sohag Governmental Hospitals, as well as exhibit the risk factors related to aborted women infection. The samples including 200 ice cream samples from which; 100 produced in small scale and 100 in large scale, and from 95 aborted women; vaginal swabs, stool, and urine were collected from each woman. The bacteriological examination and PCR were used for L. monocytogenes detection, and the virulence genes such as hlyA, Iap, PrfA, and InlA were examined by PCR, while multiplex PCR was used for the detection of biofilm genes like LuxS and flaA which have a great role in surfaces contamination in dairy processing plants. Data from aborted women was collected through standard form. 11 (5.5%) ice cream samples and 9 (9.5%) aborted women were positive for L. monocytogenes. Ice cream that produced in small scale exhibit a higher microbial load than that produced in large scale with a percentage of 8% and 3%, respectively. The significant factors that increase L. monocytogenes infection in aborted women were residence, the medical history, ice cream consumption, and contact with animals. Among the 6 examined genes in L. monocytogenes isolates; hylA gene constitutes the highest percent in both ice cream and aborted women isolates. Control measures should be applied through increased knowledge of population about listeriosis infection and using rapid diagnostic methods for effective treatment, as well as strict hygienic measures at all levels of food production chain is important for producing safe food products.
Keywords | L. monocytogenes, Virulence genes, Biofilm genes, Aborted women, Ice cream
Received | August 29, 2021; Accepted | September 05, 2021; Published | September 25, 2021
*Correspondence | Alshimaa Hassanien, Department of Zoonoses, Faculty of Veterinary Medicine, Sohag University, Egypt; Email: hassanien2008@yahoo.com
Citation | Hassanien AA, Shaker EM (2021). Virulence potential of listeria monocytogenes recovered from ice cream and aborted women samples in sohag city, egypt. Adv. Anim. Vet. Sci. 9(11): 1829-1837.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.1829.1837
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Hassanien and Shaker. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Listeria monocytogenes is a gram-positive bacterial pathogen which is considered as a ubiquitous in nature and can be isolated from the environmental sources, including water, soil, sewage, vegetables, and food (Soni et al., 2015). The main route of infection by L. monocytogenes is through ingestion of contaminated food, such as unpasteurized milk, milk products and raw unwashed vegetables (Rawool et al., 2016).
L. monocytogenes causing several outbreaks after the consumption of milk and dairy products especially ice cream (Shamloo et al., 2019). Ice cream is considered one of the most dairy products that predominates interest of populations (Abd El Fatah et al., 2015), it provides a suitable environment for L. monocytogenes growth due to its high nutritional value, low temperature, neutral pH value and long storage periods (Molla et al., 2004). L. monocytogenes can experience the cold conditions in dairy processing plants causing cold adaption of these bacteria which promote its survival in frozen dairy products such as ice cream (El shinaway et al., 2017). It has the ability to survive for 36 months in ice cream at storage temperature of −20°C without significant decrease in its number (Salazer et al., 2020).
L. monocytogenes can pose serious complications to human especially during pregnancy and neonatal infection due to its intracellular localization in the placenta, besides decreasing the cell-mediated immunity in pregnant women and in neonatal period causing fetal infections or miscarriage (Heidarzadeh et al., 2018). Pregnant women are more exposed to listeriosis than non- pregnant women by 18-times (Awofisayo et al., 2015). The World Health Organization reported that pregnancy related listeriosis represents nearly 43% of all listeria infections (Wadhwa Desai and Smith, 2017).
The detection of L. monocytogenes in food and environmental sources is a serious step toward prevention and control of L. monocytogenes outbreaks. The traditional culture method and PCR methods were used for isolation and identification of L. monocytogenes (Chen et al., 2017).
The virulent strains of L. monocytogenes are responsible for major illness and several deaths in human and animals (Pournajaf et al., 2016). L. monocytogenes pathogenesis is enhanced by a group of virulence genes (Thorat et al., 2019). Internalins encoded by InlA and InlB are surface proteins mediating the bacteria attachment and entry inside the host cells. Listeriolysin O (hylA) is cholesterol dependant toxin which facilitates the escape of bacteria from the phagocytic vacuoles and replication in the cytosol. ActA is also a surface protein that controls the motility of bacteria in the cytoplasm by polymerization of actin, and Iap is invasion associated protein. The prfA gene is considered the regulatory factor for several virulence genes such as InlA, hylA, Iap, and actA genes (Zahirnia et al., 2019).
The ability of biofilm formation is an important virulence determinant for L. monocytogenes pathogenicity and survival on surfaces in the food processing environment causing a great concern about food safety because it acts as a source of contamination (Colagiorgi et al., 2017). The flaA and LuxS genes play an important role in biofilm formation and surface attachment of L. monocytogenes. The flagellar gene (flaA) produces flagella which enhance the attachment of bacteria to biotic and abiotic surfaces (Warke et al., 2017). The LuxS gene encodes Sribosylhomocysteinase which act as a precursor of autoinducer AI-2 molecule which regulate the biofilm formation (Bonsaglia et al., 2013).
The aim of this work was to determine the occurrence of L. monocytogenes in different kinds of ice cream produced in Sohag city, Egypt and aborted women samples, and spotlight on the factors associated with aborted women infection. Also, detection of different virulence genes has a role in human public health and biofilm formation in the isolated strains.
Material and methods
Study design
Milk product (ice cream) and aborted women samples were examined for the existence of L. monocytogenes using bacteriological culture examination followed by PCR, based on specific gene detection for L. monocytogenes. All isolates of ice cream and aborted women were examined for the presence of some virulence genes using PCR and multiplex PCR. Data from patients were collected to detect the factors associated with their infection.
Collection of data and samples
Two hundred ice cream samples were purchased from diversity food premises in Sohag city; 100 samples were from small scale producers and 100 samples were from 2 different brands of large scale producers (50 for each). The human samples were collected from patients admitted to Sohag Governmental hospitals; the population under study includes 95 aborted women. 285 samples were collected from the aborted women (three samples from each woman; vaginal swab, stool and urine). Data including age, residence, knowledge about L. monocytogenes, abortion history, trimester, medical history, ice cream consumption, and contact with animals were collected from women through a standard form.
Ethical consideration
This approval was obtained from local ethical committee of Sohag University. Participation of patients and samples collection was done after their consent or their guardian consent.
Bacteriological examination of samples
Isolation and identification of L. monocytogenes from ice cream and aborted women samples was implemented according to Arslan and Özdemir, (2020).
Molecular identification of L. monocytogenes
DNA was extracted from the suspected isolates of ice cream and aborted women using QIAamp DNA, Qiagen. PCR was applied for detection of 16S rRNA gene specific for L. monocytogenes with primer sequence F: GGA CCG
Table 1: Incidence of L. monocytogenes in ice-cream samples
| Ice cream (n=200) | ||||||||
| Positive samples | Large scale (n=100) | Small scale (n=100) | ||||||
| Brand I | Brand II | |||||||
| No | % | No | % | No | % | No | % | |
| L. monocytogenes | 11 | 5.5 | 3 | 3 | 0 | 0 | 8 |
8 |
Table 2: Incidence of L. monocytogenes in aborted women samples
| Positive samples | Aborted women ( n=95) | |||||||
|
Positive women
|
Source of samples* | |||||||
| Vaginal swabs | Stool | Urine | ||||||
| No | % | No | % | No | % | No | % | |
| L. monocytogenes | 9 | 9.5 | 7 | 7.4 | 2 | 2.1 | 2 | 2.1 |
*One women has L. monocytogenes in all samples
Table 3: Characteristics and factors related to aborted women infected with L. monocytogenes
| Risk factors | Aborted women n=95 |
p value |
|||
| Examined women |
women with L. monocytogenes |
||||
| No. | % | No. | % | ||
|
Age b |
0.137 | ||||
| 18-25 | 14 | 14.7 | 2 | 2.1 | |
| 26-30 | 51 | 53.7 | 0 | 0 | |
| 31-35 | 16 | 16.8 | 2 | 2.1 | |
| >35 | 14 | 14.7 | 5 | 5.3 | |
|
Residence a |
|||||
| Rural | 34 | 35.8 | 7 | 7.4 | 0.01 |
| urban | 61 | 64.2 | 2 | 2.1 | |
|
Knowledge about L. monocytogenesb |
0.293 | ||||
| Good | 3 | 3.2 | 1 | 1.1 | |
| Fair | 11 | 11.6 | 1 | 1.1 | |
| Poor | 81 | 85.3 | 7 |
7.4 |
|
|
Abortion history b |
0.451 | ||||
|
1st time |
26 | 27.4 | 3 | 3.2 | |
|
2nd time |
48 | 50.5 | 5 | 5.3 | |
|
3rd time |
21 | 22.1 | 1 | 1.1 | |
|
Trimester b |
0.619 | ||||
|
First |
27 | 28.4 | 3 | 3.2 | |
| Second | 40 | 42.1 | 4 | 4.2 | |
| Third | 28 | 29.5 | 2 | 2.1 | |
|
Medical history * a |
21 | 22.1 | 7 | 7.4 | 0.01 |
| Renal diseases | 7 | 7.4 | 3 |
3.2 |
|
| Liver diseases | 5 | 5.3 | 0 | 0 | |
| Heart diseases | 4 | 4.2 | 1 | 1.1 | |
| Diabetes mellitus (DM) | 9 | 9.5 | 2 | 2.1 | |
| Bronchial asthma | 5 | 5.3 | 1 | 1.1 | |
| Hypertension | 7 | 7.4 | 2 | 2.1 | |
| Exposure to infection | |||||
|
Ice cream consumption a |
0.05 | ||||
| Usually | 29 | 30.5 | 6 | 6.3 | |
| Sometimes | 49 | 51.6 | 2 | 2.1 | |
| No | 17 | 17.9 | 1 | 1.1 | |
|
Contact with animals a |
|||||
| Yes | 27 | 28.4 | 6 | 6.3 | 0.01 |
| No | 68 | 71.6 | 3 |
3.2 |
|
*patients have more than one disease; a significant factors. b non significant factors
Table 4: Frequency distribution of virulence genes in L. monocytogenes isolated from ice cream and aborted women samples
|
L. monocytogenes isolates |
Virulence genes | |||||||||||
| hylA | Iap | prfA | InlA | LuxS | flaA | |||||||
| Ice cream isolates | No | % | No | % | No | % | No | % | No | % | No | % |
| Small scale (n=8) | 8 | 100 | 3 | 37.5 | 3 | 37.5 | 7 | 87.5 | 8 | 100 | 7 | 87.5 |
| Large scale (n=3) | 2 | 66.7 | 0 | 0 | 2 | 66.7 | 2 | 66.7 | 1 | 33.3 | 1 | 33.3 |
| Total (n=11) | 10 | 90.9 | 3 | 27.3 | 5 | 45.5 | 9 | 81.8 | 9 | 81.8 | 8 | 72.7 |
| Women isolates | ||||||||||||
| Vaginal swabs (n=7) | 7 | 100 | 4 | 57.1 | 5 | 71.4 | 7 | 100 | 6 | 85.7 | 4 | 57.1 |
| Stool samples (n=2) | 2 | 100 | 0 | 0 | 1 | 50 | 1 | 50 | 2 | 100 | 1 | 50 |
| Urine samples (n=2) | 1 | 50 | 1 | 50 | 2 | 100 | 0 | 0 | 1 | 50 | 2 | 100 |
| Total (n=11) | 10 | 90.9 | 5 | 45.5 | 8 | 72.7 | 8 | 72.7 | 9 | 81.8 | 7 |
63.6 |
GGG CTA ATA CCG AAT GAT AA and R: TTC ATG TAG GCG AGT TGC AGC CTA with 1200 bp according to Kumar et al. (2015) using thermal cycler from Applied biosystem. Some modifications in PCR cycle conditions were performed as following; 5 min at 94°C, followed by 35 cycles at 94°C for 45s, 60°C for 1 min, and 72°C for 1 min, and a final extension for 10 min at 72°C. The product of PCR was examined in 1% agarose gel electrophoresis with ethidium bromide and photographed by light transilluminator from Biometra (Germany).
Detection of virulence genes by PCR
The L. monocytogenes strains of both ice cream and aborted women were examined for the presence of some virulence genes according to Aziz and Mohamed, (2020) with modifications in the cycling conditions. These virulence genes were including hlyA gene with specific primer sequence F: GCA-TCT-GCA-TTC-AAT-AAA-GA and R: TGT-CAC-TGC-ATC-TCC-GTG-GT with 174 bp and cycling condition: primary denaturation for 4 min at 95˚C, 35 cycles at 95˚C for 30 sec, followed by 60˚C for 30 sec, 72˚C for 1 min and final extension for 5 min at 72˚C. The Iap gene with primer sequence F: CTG CTT GAG CGT TCA TGT CTC ATC CCC C and R: CAT GGG TTT CAC TCT CCT TCT AC with 131 bp and cycling condition: 5 min at 94˚C, 35 cycles at 94˚C for 30 sec, followed by 55˚C for 30sec, 72˚C for 1 min, and 72˚C for 10 min. The prfA gene with primer sequence F: TCT-CCG-AGC-AAC-CTC-GGA-ACC and R: TGG-ATT-GAC-AAA-ATG-GAA-CA with 1052 bp and cycling condition: 95˚C for 5 min, 30 cycles for 30 sec at 55˚C, followed by 72˚C for 2 min, and final extension at 72˚C for 5 min. InlA gene with primer sequence F: ACG AGT AAC GGG ACA AAT GC and R: CCC GAC AGT GGT GCT AGA TT with 800 bp and cycling condition: 2 min at 94˚C, 30 cycles for 20 sec at 94˚C, followed by 55˚C for 30 sec, 72˚C for 50 sec, and final extension for 5 min at 72˚C.
Detection of virulence genes by multiplex PCR
LuxS and flaA genes were detected by multiplex PCR according to Thorat et al. (2019) with some modifications in the cycling condition using LuxS primer sequence F: GGA AATGCCAGCGCTACACTCTTT and R: ATTGCATGCAGGAACTTC TGT CGC with 208 bp and flaA gene sequence F: GCGCAAGAACGTTTAGCATCTGGT and R: TTGAGT AGCAGCACCTGTAGCAGT with 363 bp. The PCR cycling condition was initial denatruation for 5 min at 95˚C, followed by 35 cycles at 94˚C for 15 sec, 1 min at 56˚C and 45 sec at 72˚C and final extension at 72˚C for 10 min.
Statistical analysis
SPSS 14 (SPSS, USA) was used to detect the relation between L. monocytogenes infection and women characteristics.
Results
L. monocytogenes was investigated using 16S rRNA gene in ice cream and aborted women samples (Figure 1). Out of 200 ice cream samples, 11 (5.5%) were harbor L. monocytogenes. Ice cream sold in small scale producers represented a higher infection rate (8%) than large scale producers (3%) which were positive only for one brand (Table 1). Among 95 aborted women; 9 (9.5%) women were positive for L.monocytogenes. Eleven isolates were recovered from the different 9 aborted women samples, only one aborted woman was carried L. monocytogenes in all body samples and the vaginal swabs were represented the highest load of infection in 7 samples, followed by 2 in stool samples and 2 in urine samples (Table 2).
The aborted women characteristics in Table 3 showed that most of positive L. monocytogenes cases were detected in women aged >35 years in a percentage of 35.3 %, followed by women aged 18-25 and 31-35 with infection rate of 2.1% , while women aged 26-30 years gave negative results. Women live in rural communities (7.4%) represented higher infection rate than those live in urban area (2.1%). The majority of the infected women had poor knowledge about L. monocytogenes. Aborted women for second time and those in second trimester of pregnancy reported the highest infection rate in a percentage of 5.3% and 4.2%, respectively. Women have suffered from chronic diseases such as renal, liver, and heart diseases; diabetes mellitus, bronchial asthma, and hypertension were more susceptible to L. monocytogenes infection with a percentage of 7.4%. Based on the exposure to infection; women eating ice-cream (6.3%), and women in contact with animals (6.3%) showed high susceptibility to the infection. Regarding factors associated with L. monocytogenes infection; residence, the medical history of aborted women, consumption of ice cream, and contact with animals significantly associated with infection, while age, range of knowledge about L. monocytogenes, abortion history, and trimester reported no significant relation to infection.
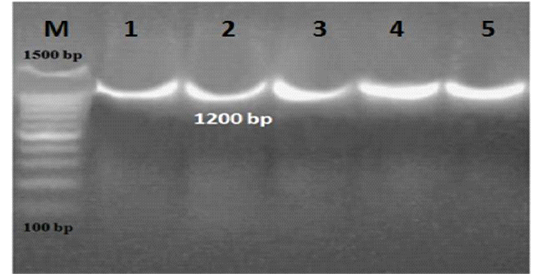
Figure 1: PCR results for 16S rRNA gene in ice cream and aborted women samples. M: marker, Lane 1-5: positive for L. monocytogenes
Referring to the L. monocytogenes virulence genes; PCR and multiplex PCR were used for detection of 6 genes (hylA, Iap, prfA, InlA, LuxS and flaA) in ice cream and aborted women isolates, and found that hylA gene was present with the highest percent among all the examined genes. Three (27.3%) of 11 ice cream isolates and 2 (22.2%) out of 9 aborted women isolates were positive for all the examined virulence genes (Table 4, Figure 2, 3).
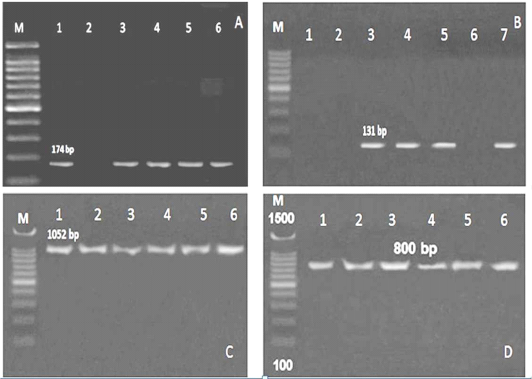
Figure 2: PCR results for virulence genes detected in L. monocytogenes isolated from ice cream and aborted women samples A: M: marker, Lane 1,3,4,5 and 6: positive for hylA gene, Lane 2: negative, B: Lane 1, 2 and 6: negative for Iap gene, Lane 3, 4,5 and 7: positive, C:Lane 1-6: positive for prfA gene, D: Lane 1-6: positive for InlA gene
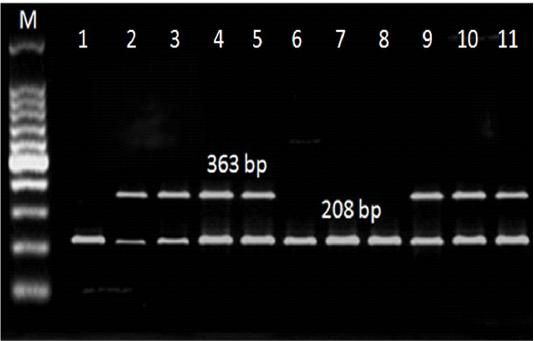
Figure 3: Multiplex PCR results for LuxS and flaA genes detected in L. monocytogenes isolated from ice cream and aborted women samples. M: marker, at 208 bp: Lane 1-11: positive for LuxS gene. At 363 bp: Lane 1,6,7, and 8: negative for flaA gene, Lane 2,3,4,5,9,10, and 11: positive.
Discussion
Listeriosis infection is associated with contaminated food consumption such as ready to eat meat products, unpasteurized milk, seafood and ice cream. The contamination of food may occur during food processing stages such as preparation, packaging, handling, transportation, and storage (Garner and Kathariou, 2016). Ice cream is a popular and delicious food where milk should be pasteurized before freezing (Yousef et al., 2020).
L. monocytogenes was detected by PCR using 16S rRNA gene in ice cream samples with a percentage of 5.5% (Table 1). Nearly similar results were reported by Adil et al. (2017) who detected L. monocytogenes in 6% of ice cream samples, while high incidence (12.3%) was detected by Windrantz and Arias (2000) and low incidence (2%) was obtained by Youssef et al. (2020). In the contrary, Abrahão et al. (2008) cannot detect L. monocytogenes in ice cream samples. Two types of ice cream were examined; the first one produced in a large scale which manufactured from pasteurized milk and sold packaged in different markets and groceries with several brands, and we studied two brands only. The second one produced in a small scale with unpasteurized milk and sold unpackaged in different shops. L. monocytogenes was detected in 8% of small scale samples, and in 3% of large scale samples in brand I, while brand II reported negative results. Increased infection in small scale ice cream may be attributed to the source of milk which used in production and other raw materials, also it exposed to several sources of contamination during processing, transportation and handling from food handlers, contaminated equipment and environment. The presence of L. monocytogenes in one brand of the large scale ice cream may be resulted from post pasteurization contamination of milk in the factory. Therefore, the major points for the control of pathogens are pasteurization of milk, freezing, and hardening steps, but the latter 2 not decrease the level of L. monocytogenes (Abrahão et al., 2008). As a result of the post pasteurization contamination of ice cream; L. monocytogenes can survive in ice cream due to its neutral pH which enables its growth. Also, it can grow at chilled temperatures at 4, 8, 12 and 16˚C and it survive at a static freezing temperature at -5, -15, -23, and -33˚C without any decrease in the bacterial numbers up to 90 days (Gougouli et al., 2008).
Although L. monocytogenes is uncommon microorganism to cause diseases for the general population, it capable of inducing a wide range of illness in pregnant women, neonates, elderly people, and immunocompromised individuals (Mehmood et al., 2017). Pregnant women are more susceptible to infection nearly 18 times larger than the general population due to the natural immunosuppression of pregnancy (Mateus et al., 2013).
L. monocytogenes was detected in 9 (9.5%) women of 95 aborted women. Eleven isolates were recovered from 9 patients, out of these, 7 of vaginal swabs, 2 urine samples and 2 of stool samples (Table 2). Different results were mentioned by Eslami et al. (2014) and Al-Mayahi et al. (2020) who detected L. monocytogenes in 16.7% and 4.8% of aborted women, respectively. The variation in results may be related to women immunity, medical history, food habit, geographic region, contact with infected animals, and rang of knowledge about disease transmission. As shown in Table 3, out of 9 infected women with L. monocytogenes; 5 (5.3%) aged >35 years, 7 (7.4%) live in rural areas, 7 (7.4%) represented poor knowledge about listeriosis, 7 (7.4%) had chronic diseases, 4 (4.2%) aborted in the second trimester, and 5(5.3%) aborted for the second time. Our results revealed that aborted women have L. monocytogenes exhibit influenza like symptoms such as myalgia, headache, fever below 39˚C, and diarrhoea. The unexplained fever and nonspecific symptoms of listeriosis during pregnancy make it difficult to be diagnosed which leads to abortion, especially in the second trimester (Pourkaveh et al., 2016).
The risk factors significantly associated with women infection with L. monocytogenes include residence (p< 0.01), medical history (p< 0.01), consumption of ice cream (p< 0.05), and contact with animals (p< 0.01) (Table 3). This significance may be attributed to the decreased immunity of aborted women due to chronic diseases beside pregnancy which makes these women more susceptible to infection from different sources such as, contaminated food, infected farm animals, untreated water and soil, especially in rural communities in which women more exposed to infection in agriculture work, and home activities including rearing of farm animals and birds. The food habits such as eating unpackaged food, and under cooked food, and low knowledge about diseases transmission may increase the chance of disease occurrence in the community. Therefore, increase the awareness of people about disease source, mode of transmission, severity, and the preventive measures may change their behaviour toward positive practice and reduce the infection with diseases.
Infection with L. monocytogenes depends on the immunity of the infected subjects, infection dose and the type of virulence genes in the bacterial strain. However, the susceptibility for infection was increased in case of immunodeficiency people (Wang et al., 2021). Once L. monocytogenes invade the human body by oral administration, it reaches to the small intestine mucosa, and then invades other organs through the circulation and lymph nodes. Different proteins were secreted by L. monocytogenes to attack the host cells such as internalins. After entrance inside the cell, it releases phospholipases and listeriolysins to dissolve the membrane of the phagocytic vacuoles to survive in the cells. When L. monocytigenes reach the cytoplasm, it proliferates and enhances the development of actin filaments, and then penetrates the cytoplasm into the plasma membrane and intercellular diffusion to infect the neighbouring cells. The intercellular circulation allows L. monocytogenes to escape from human T cell immunity and move from one cell to another and infect other tissues and organs especially placenta tissue in case of pregnancy (Charlier et al., 2014).
L. monocytogenes can form biofilm which allows bacteria to resist the environmental stress such as varied temperature, dehydration, and treatment with sanitizing and antimicrobial agents. Formation of biofilm on surfaces during food processing considered an important factor of L. monocytogenes survival (Barbosa et al., 2013). Detection of biofilm genes such as LuxS and flaA are a determinant genes of L. monocytogenes pathogenicity (Warke et al., 2017).
PCR and multiplex PCR were used to detect some virulence genes in L. monocytogenes isolates using specific primers. The isolated strains of L. monocytogenes from ice cream and aborted women harbour some virulence genes such as hylA, Iap, prfA, InlA, LuxS, and flaA (Table 4). Consistent with results that reported by Throat et al. (2019) who detected plcA, hlyA, actA, prfA, inlC, inlJ, luxS and flaA genes in L. monocytogenes isolated from different sources as human, food, animals, and mosquitoes. Listeriolysin O (LLO) is considered the most virulence factor of L. monocytogenes which encoded by hylA gene and present only in the virulence strains (AL-Ashmawy et al., 2014). The most prominent gene in both women and ice cream isolates of L. monocytogenes was hylA gene which indicated that theses isolates is virulent strains.
Ice cream isolates of L. monocytogenes harbour hylA gene with a percentage of 90.9%, followed by LuxS and InlA with a rate of 81.8%, followed by flaA (72.7), prfA (45.5%), and Iap (57.1). Consistent with Abd El Tawab et al. (2015) who detected InlA, InlB, hlyA and prfA genes in L. monocytogenes isolated from ice cream samples. Presence of pathogenic strains of L. monocytogenes in ice cream samples revealed that freezing temperature not affects on its survival and pathogenicity. We cannot depend on only storage of dairy products at refrigeration or freezing temperatures to control L. monocytogenes (Bucur et al., 2018). Therefore in dairy industry; using safe materials and following hygienic measures in all steps of manufacturing, packaging, transportation and handling is important to produce safe products.
Aborted women isolates of L. monocytogenes have hylA gene with a percentage of 90.9%, followed by LuxS gene (81.8%), prfA and InlA (72.7%), flaA (63.6%), and Iap (45.5%). These results goes parallel with Kaur et al. (2007) who detected prfA, hylA,and Iap genes in L. monocytogenes isolates of women with spontaneous abortion, and Shaker and Hassanien (2015) who detected hylA, and InlA genes. Presence of these genes in the isolated strains increases L. monocytogenes attachment, invasion, development and survival inside the human cells which enhance its pathogenicity especially in immunodeficiency patients and it is a good indicator of the virulence level of L. monocytogenes (Liu et al., 2007).
Conclusion
Pathogenic strains of L. monocytogenes were detected in ice cream although it stored at freezing temperature which pose a great threat to consumers. Contamination of large scale and small scale produced ice cream indicated that surveillance and control measures should be applied at all food processing stages. Detection of L. monocytogenes and the risk factors associated with infection in aborted women is important for effective treatment and control of listeriosis infection. Detection of virulence genes in the isolated strains is an important indication for its pathogenicity and survival in the surfaces through biofilm formation.
Conflict of interest
None.
Acknowledgments
Authors thank the molecular biology research unit in Animal Health Research Institute, El-Giza, Egypt (EGAC /ISO/ 17025/2017), and the medical staff in hospitals for their help in this study.
authors contribution
AAH and EMS contributed equally in designing the study, samples and data collection, literature search, laboratory work, data analysis, writing and preparation of the manuscript. Both authors approved the final manuscript.
References





