Advances in Animal and Veterinary Sciences
Research Article
Morphometry and Biometry of Gastrointestinal Tract of Indigenous Sheep
Nasrin Sultana1*, Rafiqul Islam1, Marzia Afrose2, Nure Jannat3
1Department of Anatomy and Histology, Bangladesh Agricultural University, Bangladesh; 2Department of Livestock Science and Veterinary Medicine, Bangabandhu Sheikh MujiburRahman Science and Technology University, Bangladesh; 3Department of Anatomy and Histology, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Bangladesh.
Abstract | Sheep gastrointestinal tract (GIT) plays a vital role in storage and microbial digestion of ingested feedstuffs which is indispensible for fulfilling the energy requirement and maintenance of body functionality. The histo-morphometry and biometry of GIT of indigenous sheep were studied. Samples were collected from the GIT i.e. rumen, reticulum, cecum and colon of five indigenous sheep in Bangladesh. The samples were collected immediately after slaughtering. The samples were stained with Hematoxylin and Eosin. The tissues were examined under light microscope and photographs were taken by digital camera.The biometric measurements of different parts of the GIT were performed using calibrated stage micrometer. Morphologically, the GIT wall was comprised of mucosa, submucosa, muscularis and serosa. The mucosa of rumen and reticulum revealed numerous papillae. Each papilla lined by stratified squamous epithelium inrumen and reticulum. The average length and width of papillae were more in rumen than in reticulum. The lamina muscularis was absent in rumen. The submucosa comprised of irregular connective tissues. The mucosa of cecum and colon throwned into numerous leaf shaped folds lined by simple columnar epithelia with large number of goblet cells. The lamina propria filled with crypts of lieberkuhn in cecum and colon. The diameter of crypts of lieberkuhn was more in cecum than in colon which was statistically non significant. Tunica muscularis of the GIT composed of two layers of smooth muscle bundles in rumen, reticulum, cecum and colon. The average length of tunica muscularis was the highest in rumen and the lowest in cecum. The serosa consisted of loose connective tissues and mesothelium. Data obtained frombiometric study of GIT were recorded in sheep. In conclusion, the morphometric and biometric study of GIT provide necessary information for the practicing veterinary gastroenterologist and researchers.
Keywords | Sheep, GIT, Morphometry, Biometry
Received | April 01, 2021; Accepted | April 15, 2021; Published | September 15, 2021
*Correspondence | Nasrin Sultana, Department of Anatomy and Histology, Bangladesh Agricultural University, Bangladesh; Email: nasrin.sultana@bau.edu.bd
Citation | Sultana N, Islam R, Afrose M, Jannat N (2021). Morphometry and biometry of gastrointestinal tract of indigenous sheep. Adv. Anim. Vet. Sci. 9(10): 1739-1744.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1739.1744
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Sultana et al., This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The gastrointestinal tract (GIT) is the part of digestive system which extends from the esophagus to the anus in ruminant and equine (Getty, 1975). It consists of esophagus, stomach, intestine and anus. Sheep (Ovis aries) are among small ruminant having four chambered stomach (Rumen, Reticulum, Omasum and Abomasum) as like other mammals’ ruminant (Medjekal et al., 2021). The digestive system is the only system, which satisfies the energy need of the body through absorption of nutrients and thus it makes the powerful relation with nature by digesting various types of feed, which are digested to specific animals (AL-Samawy et al., 2018). The digestive system constitutes a series of tubular organs and associated glands whose main function is to break down the ingested food materials into smaller units that can be absorbed and utilized for the maintenance of the organism (Yıldırım et al., 2014).
The structural and functional abilities in specific regions of the GIT impart differential functional capabilities to these segments. Optimum health and efficacious functional abilies of the GIT are indispensible factors for ensuring productive performance like optimum growth with quality milk and meat production (Celi et al., 2017). Sheep allowed to roam and scavenge for food ingest various types of non food foreign particles (Otsyina et al., 2015). These indigestible foreign materials cause numerous histological changes in the wall of rumen of sheep (Otsyina et al., 2017). A large number of researches were held on the adaptations of histomorphology of the GIT in sheep. Different feeding strategies and lactation length altered the tissue histology of the GIT i.e. rumen in lamb (Álvarez-Rodríguez et al., 2012). Parasitic infections i.e. nematode infections are major setback for sheep rearing causing great production loss and fatality (Starling et al., 2019). These parasites alter tissue orientations of the GIT in sheep (Amarante et al., 2007, Satish et al., 2018). Nutritional manipulation in feeding trials changes the morphologic responses of the GIT i.e. rumen in cow and sheep (Khodakaram-tafti and Hashemnia 2017, Resende-junior et al., 2006).
Like other ruminants’ species, morphometric and biometric features of the GIT have been studied in goat and camel (Bello and Danmaigoro 2019, Mohamed et al., 2018). Despite the importance of the histomorphology and biometry of the GIT in education, researches, and disease diagnosis, the literature on the histomorphology of the GIT wall of sheep is insufficient to study the adaptations on the GIT of sheep, though Dobson et al. (1995) describe only the histology of rumen wall. AL-Samawy et al. (2018) conducted a study and compare the histomorphology of the GIT i.e. colon in sheep and goat. In the above mentioned literatures, histomorphology of only some parts of the GIT i.e. rumen and colon are very briefly studied in sheep. However, the detailed histomorphology and biometric characteristics of the GIT in indigenous sheep have never been studied. Better understanding of the structure of sheep GIT is crucial for enhancing production through proper care and feeding management. Therefore, the present study was carried out to describe the histomorphology and biometry of wall of the GIT in indigenous sheep.
MATERIALS AND METHODS
Ethical approval
The present experimental procedures, animal handlings, and sample collections were approved by the guidelines for the care and use of animals as established by Animal welfare and Experimentation Ethics Committee, Bangladesh Agricultural University, Bangladesh (AWEEC/BAU/2020(24)).
Experimental animals
The study was carried out on five 1-year-old indigenous male sheep (Ovis aries L). The study was conducted in the Department of Anatomy andHistology, Faculty of Veterinary Science, Bangladesh Agricultural University, Bangladesh. The sheep had no developmental disorders and detectable diseases that may cause any drawback to study the histo-morphometry and biometry of the GIT in sheep.
Tissue sampling
The animals were slaughtered using halal method to collect the target samples i.e. rumen, reticulum, cecum, and colon of the GIT. The samples were collected immediately after slaughtering with the help of sterile scalpel and scissors through ventral abdominal dissection, which were free from pathological lesions. The collected tissue samples were washed with phosphate-buffered saline and then stored in formalin for morphometric and biometric study.
Histo-morphometric study
For histological studies the tissues obtained from the GIT of sheep were fixed in the “Bouins fluid” (Gridley, 1960) for 24 hours. Then the tissues were dehydrated in the series of ascending grade of alcohol followed by clearing in three changes in xylene. After that, the tissues were then infiltrated with different grades of melted paraffin (49 °C, 55 °C, and 58 °C) in the oven. The tissues were then embedded inmelted paraffin and finally the sections were cut at 5-6μm (micrometer) thickness using sliding microtome (MIC 509, Euromex, Japan). After cutting, the sections were floated on luke-warm water in a floatation bath at 37ºC for stretching. Then the sections were mounted on clean slides using an adhesive i.e. egg albumin and dried on a slide warmer at 37ºC. The sections were stained using Mayer’s Hematoxylin and Eosin (H & E) for histomorphometrical analyses.
Photomicrographs
The histological structures of the tissues (rumen, reticulum, cecum and colon) were examined using light microscope under low (×10) and high (×40) magnifications. The necessary photographs were taken from the selected specimens using photomicroscope (U-LH50HG, Olympus Corporation, Tokyo, Japan) for better illustration of the obtained results. For each section, 10 randomly selected fields were captured for proper histomorphometric evaluation.
Biometric study
The biometric measurements of different histological structures of the tissues were performed using calibrated stage micrometer in μm (micrometer). Twenty sections (five from each tissue) were biometrically evaluated from five indigenous sheep. The length and width of papillae (rumen and reticulum), crypts of lieberkuhn (cecum and colon), and tunica muscularis (rumen, reticulum, cecum and colon) were considered for biometric measurement. These parameters were measured from ten fields of each section and the mean value was taken for further statistical analysis.
Statistical Analysis
The results of biometric measurement were analyzed using SPSS software (IBM SPSS Statistics 22). The normality of the data set was evaluated by Shapiro-Wilk test. Obtained results were presented as mean ± standard error of mean (SEM). Differences among the different organ samples were compared using one-way ANOVA with post-hoc Duncan’s multiple range test where P<0.05 were considered significant.
RESULTS
Histo-morphometry of GIT in sheep
The histomorphology of rumen and reticulum of sheep was presented in a Figure (1 and 2). Histologically, the wall of rumen was composed of four layers i.e. tunica mucosa, tunica submucosa, muscularis externa and tunica serosa. The mucosa of rumen of sheep had papillae of different shapes and size i.e. long papillae and short papillae. Long papillae were found as tongue shape and short papillae were conical in shape. Each papilla was lined by stratified squamous epithelium which was rested on a lamina propria of loose connective tissue. The tunica submucosa was comprised of mostly collagen fibers, blood vessels, and nerves. The mucosa of rumen was combined with submucosa as there was no lamina muscularis. Tunica muscularis was composed of two layers of smooth muscle bundles i.e. inner circular and outer longitudinal layers. The myenteric plexus was located between the inner and outer layers of smooth muscles. The tunica serosa was composed of loose connective tissue with anouter lining of mesothelium.
The mucosa of reticulum was surrounded by numerous conical shaped papillae or laminae in sheep. The composition of lining epithelium of each lamina was similar with that of rumen. The lamina muscularis of reticulum was present in the core of papilla along with lamina propria. Tunica muscularis was composed of two layers of smooth muscle bundles. The tunica serosa was composed of loose connective tissue and mesothelium.
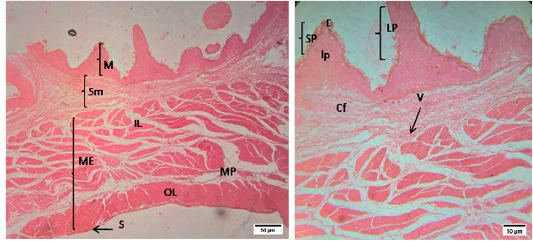
Figure 1: Representative photomicrographs of the rumen in sheep. A(H&E, 10X):M-Mucosa, Sm-Submucosa, ME-Muscularis Externa, IL- inner layer, OL- outer layer, MP- Myenteric plexus, S-Serosa; B(H&E, 40X): E-Epithelium, lp-lamina propria, SP- short papillae, LP- long papillae, Cf- Collagen fibers, V-Vessels.
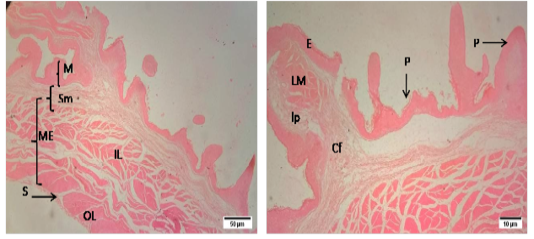
Figure 2: Representative photomicrographs of the reticulum in sheep. A(H&E, 10X): M-Mucosa, Sm-Submucosa, ME-Muscularis Externa, IL- inner layer, OL- outer layer, S-Serosa. B(H&E, 10X): E-Epithelium, lp-lamina propria, P- papillae,, Cf- Collagen fibers, V-Vessels.
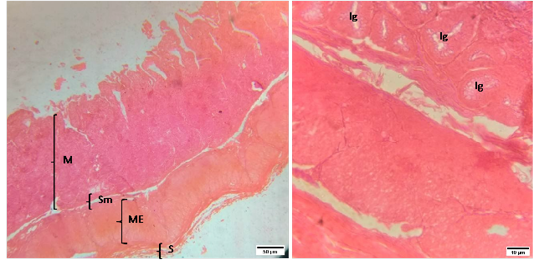
Figure 3: Representative photomicrographs of the cecum in sheep. A(H&E, 10X): M-Mucosa, Sm-Submucosa, ME-Muscularis Externa, S-Serosa. B(H&E, 40X): Ig-Intestinal glands.
The histomorphology of cecum and colon of sheep were shown in Figure (3 and 4).The morphology of cecal wall was comprised of four layers i.e. tunica mucosa, tunica submucosa, muscularis externa and tunica serosa. The tunica mucosa of cecum was thrown into numerous leaf shaped folds lined by simple columnar epithelia with large number of goblet cells. The lamina propria was filled with intestinal glands or crypts of lieberkuhn rested on a layer of smooth muscle, lamina muscularis. The tunica submucosa of cecum was made up of dense connective tissue. The lymphatic nodules were scattered throughout the mucosa. The muscularis externa was composed of inner circular and outer longitudinal layers of smooth muscle separated by a narrow connective tissue layer.
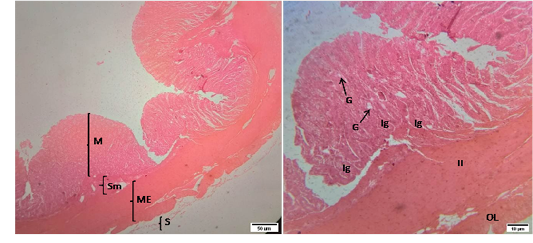
Figure 4: Representative photomicrographs of the colon in sheep. A(H&E, 10X): M-Mucosa, Sm-Submucosa, ME-Muscularis Externa, S-Serosa. B(H&E, 40X): G-Goblet cell, Ig –Intestinal glands, IL-Inner circular muscularis externa, OL- Outer longitudinal muscularis externa.
The mucosa of colon was similar with that of cecum except the number of goblet cells which weremore than those in cecum. The intestinal glands extended into the submucosa. The submucosa of colon was infiltrated with lymphatic tissues. The muscularis externa of colon revealed as inner circular and outer longitudinal layer of smooth muscles. The outermost layer of colon wasthe tunica serosa that was comprised of loose connective tissue covered by mesothelium.
Biometry of GIT in sheep
In the present study, data obtained from the biometric parameters of GIT of sheep were presented in Table (1). The average length of papillae was (1753.4±7.7µm) was more in rumen than in reticulum (1151.6±5.9µm) of sheep. The average width of papillae of rumen (718.2±8.6µm)was also more than in reticulum (315±9.1µm) of sheep. The length of tunica muscularis or muscularis externa was1213.6±8.1µm, 1114±8.1µm, 605.4±8.8µm and 707.4±8.4µm in rumen, reticulum, cecum and colon respectively. The diameter of crypts of lieberkuhn was28.6±3.6µm and 27.4±4.4µm in cecum and colon respectively.
DISCUSSION
The digestive system is composed of the mouth, esophagus, stomach, small and large intestines, and anus. Sheep, like other ruminants, have four chambers i.e. reticulum, rumen, omasum, and abomasum in stomach. Among them, the rumen serves as the primary site for microbial fermentation of ingested feed. The reticulum largely acts to further mechanical dissociation of digesta following microbial breakdown. Actually, there is no distinct division between the rumen and the reticulum, they are often

Table 1: Biometric properties of the GIT i.e. rumen, reticulum, cecum and colon of indigenous sheep (n=5).
referred to together (reticulo-rumen). The ruminant large intestine comprised of the cecum and the colon (Jennings and Premanandan 2017). In ruminants, approximately 10-15% of the animal’s energy requirement is supplied through microbes in the caecum (Krajmalnik-Brown et al., 2012). Physiological factors that cause animal variation in digestive function are evaluated as possible selection traits to achieve improved growth, fattening performance and feed-use efficiency by sheep (Hegarty 2004). Significant differences in digestive function existed between and within breeds (Hegarty 2004), which may cause differences in GIT characteristics between and within breeds. Like functional differences, differences between and within sheep breeds in terms of morphology and physiology are nutritionally important in judging local sheep breeds, which is important not only for preserving genetic diversity, but also for sustainable rural development in developing countries (Carneiro et al., 2020).
Histo-morphometry of GIT in sheep
The histological structures of ruminal and reticular walls were made up of four layers i.e. tunica mucosa, tunica submucosa, muscularis externa and tunica serosa in sheep. Structural differences of ruminal and reticular walls are observed in different physiological and pathological conditions in animals.The morphological changes may occur in individual layers of the wall of rumen and reticulum in animals. In methane emissions, the morphology i.e. size of rumen and reticulum is changedin sheep (Waite et al., 2019). Nutritional manipulation can alter the rumen epithelial morphological responses in sheep and cow (Resende-junior et al., 2019). Plastic bags in the rumen causes numerous histopathological changes i.e. atrophy, ulceration, erosion and disruptions of the stratified epithelial layer of the papillae of rumen. In addition to these, other changes like parakeratosis, hyperkeratosis, prominent rete pegs, oedema and severe hydropic degeneration are also found in the mucosa of rumen. There are increased mononuclear cells infiltrations, increase in the number of lymphatic vessels and lymphangiectasis in the submucosa and oedema in the muscularis and serosal layers (Otsyina et al., 2017). The width of the ruminal papillae varied with different diets i.e. corn silage or corn silage and concentrate in sheep. In this study, mitotic index were used to evaluate the responses of epithelial cells of rumen. Mitotic index was higher in the diet rich with corn silage (Lima et al., 2015). So, the present study results may contribute to evaluate the histological alterations occured in different layers of rumen and reticulum wall in sheep.
In the present research, the morphology of cecum and colon wall was composed of four layers i.e. tunica mucosa, tunica submucosa, muscularis externa and tunica serosa.This result is in agreement with an observation reported in camelid (Mohamed et al., 2018). In this study, the authors described the histological structures of cecum and colon wall in camel. They explained the thickness of different layers of cecum and colon walls in camel. In the present study, the composition of all histological layers of cecum and colon walls was described in sheep. The epithelial characteristics (i.e. tall columnar epithelia with goblet cells) of colon are similar in sheep and goat. But, there is a difference between the mucosal folds of colon in sheep and goat (AL-Samawy et al., 2018). In sheep, the mucosal folds appeared long leaf-shaped arranged in a zig-zag pattern whereas, in goat mucosal folds appeared as short and blunt. The composition of submucosa, muscularis externa, and serosa is identical in sheep and goat but there is an inequality in the thickness of these layers in sheep and goat (AL-Samawy et al., 2018). The current knowledge in histological structures of intestinal wall and its composition will help to evaluate the morphological alterations of intestine occurred in different pathological conditions of sheep. In esophagotomiasis, the intestinal wall was greatly thickened and edematous and the nodules were seen projecting into the lumen but the mucosal surface remained intact. In addition to these changes, necrotic areas are also observed (Satish et al., 2018). In intestinal coccidiosis, papillary hyperplasia of villi epithelium is observed with the presence of intracytoplasmic developmental stages of the parasite and infiltration of inflammatory cells (Khodakaram-tafti and Hashemnia 2017).
Biometry of GIT in sheep
Diet and time related changes in the surface area, length and width of papillae of rumen have been noticed in ruminants (Dieho et al., 2016). Recently, knowledge about the biometric measurements of GIT is limited in sheep. In this study, the average length and width of papillae were reported in rumen and reticulum of sheep. The length of tunica muscularis was recorded in rumen, reticulum, cecum and colon of sheep. Among these, the average length of tunica muscularis is highest in rumen and lowest in cecum. This study is in agreement with other study in camels where the mean thickness of muscularis externa in colon is more than that in cecum (694.7±37.4) μm and (513.9±46.3) μm respectively (Mohamed et al., 2018). The diameter of crypts of lieberkuhn was more in the cecum than in the colon of adult sheep. The current knowledge of the rumen and reticular papillae (papilla length and width) will help to identify any morphological adaptations occurred in ruminal and reticular papillae in sheep. The current study recommends more studies on the different parts of the digestive system in sheep and compare it with others ruminants.
Conclusion
The local production of sheep will provide milk, skin and wool for the farmers and a good source of revenue to the state of government. In the present study, histological structures and biometry of GIT were described in sheep. The present approach will play a major role in veterinary education and researches i.e. for studying undergraduate and graduate students and to understand the adaptations in the morphology and biometry of GIT in response to different pathological conditions respectively. This study presents clinically useful information for practicing veterinary gastroenterologists. It would also help to a large extent in establishing the characterization of the sheep populations in different physiological and pathological conditions.
Acknowledgements
Technicians of the department of Anatomy and Histology, Bangladesh Agricultural University, Bangladesh are thanked for their assistance in performing the experiment.
Author’s contribution
NS conceptualized and designed the study. RI and MA performed the laboratory works. NJ analyzed the data. NS drafted the manuscript.
Conflicts of interest
There is no conflict of interest among the authors.
REFERENCES





