Advances in Animal and Veterinary Sciences
Review Article
Avian Aspergillosis: A Potential Occupational Zoonotic Mycosis Especially in Egypt
Wafaa A. Abd El-Ghany
Poultry Diseases Department, Faculty of Veterinary Medicine, Cairo University, 12211, Giza, Egypt.
Abstract | Poultry production systems show great interest especially in the last decade. However, using intensive management systems leads to increasing the incidence of infectious diseases. One of the most dangerous infectious fungal diseases is aspergillosis. This disease has a negative impact on the poultry industry with severe economic losses. There are several species of Aspergillus fungi that affect birds of different ages. Poor environment and hygienic conditions surrounding birds in the hatcheries or farms are important sources of infection. Affected birds with Aspergillus species show respiratory signs and lesions as well as brain and skin affections. Aspergillosis is an important occupational zoonotic mycosis that affects mainly persons and workers who are in direct contact with the infected birds. The respiratory picture of aspergillosis in human s is usually very severe especially in immunocompromised patients. Diagnosis of aspergillosis depends on conventional isolation and identification of the causative fungi, serological detection of antibodies or use molecular identification techniques. The application of proper hygienic measures with good environment al conditions surrounding birds is very critical to prevent such infection. Treatment of affected birds using specific antifungal drugs is useless especially in severe cases or in later stages. Human s should take all sanitary precautions during handling with birds and the surroundings. Affected persons could be treated using specific antifungal preparations. Therefore this review article spotlight the present situation of aspergillosis all over the world as well as in Egypt . The article focuses on the fungi, susceptibility, and transmission of infection, clinical picture in birds, human infection, diagnosis as well as prevention and control measures.
Keywords | Aspergillus species, Birds, Egypt, Human, Zoonosis
Received | April 20, 2021; Accepted | July 30, 2021; Published | August 15, 2021
*Correspondence | Wafaa A. Abd El-Ghany, Poultry Diseases Department, Faculty of Veterinary Medicine, Cairo University, 12211, Giza, Egypt; Email: wafaa.ghany@yahoo
Citation |Abd El-Ghany WA (2021). Avian aspergillosis: A potential occupational zoonotic mycosis especially in Egypt. Adv. Anim. Vet. Sci. 9(10): 1564-1575.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1564.1575
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 El-Ghany et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
One of the main important constrain facing the poultry industry is fungal affection. Aspergillosis has been considered the most common respiratory fungal infection of poultry (Arné et al., 2011). The disease causes severe economic problems in the commercial poultry production system including; high mortalities especially in young’s, decreasing the growth rates, immunosuppression, and increasing in the condemnation rates during slaughtering (Lupo et al., 2010; Seyedmousavi et al., 2015). Aspergillus species are regarded as saprophytic organisms that mostly present in the soil, decomposed plants, grains, and seeds (Fang and Latgé, 2018). However, some species of this fungus are highly pathogenic. Aspergillus fumigatus (A. fumigatus) is the most common and frequently isolated pathogenic species for birds (Arné et al., 2011; Cheng et al., 2020), followed by A. flavus (Martin et al., 2007) and A. niger (Saleh et al., 2011). Nearly all avian species and ages are susceptible to Aspergillus infections (Pazhanivel et al., 2018; Talbot et al., 2018; Hauck et al., 2020; Melo et al., 2019, 2020). The disease in birds is mainly associated with the respiratory form (Nururrozi et al., 2020). However, other pictures of Aspergillus infections were also recorded in the nervous system and skin of the affected birds (Stoute et al., 2009; Beernaert et al., 2010).
There are over 300 species of Aspergillus with human public health importance, however, A. fumigatus, A. flavus, A. niger and A. terreus, are mostly responsible for human infections (Oliveira et al., 2015). Other species like A. lentulus, A. thermomutatus, A. pseudofischeri and A. felis were also recorded in human cases (Lamoth, 2016). The disease in human s is associated with severe pulmonary affection especially in immune-compromised persons (Zilberberg et al., 2018). Aspergillosis is regarded as an occupational zoonotic disease as a human can gain infection by direct and indirect contact with infected avian species. Workers in poultry farms are at high risk of Aspergillus infection as they can be exposed to high levels of dust-containing spores during handling and processing of contaminated litters and materials (Sabino et al., 2012; Mubarak and Mohamed, 2017). From the public health point of view, aspergillosis has been incriminated as one of the most feared opportunistic human infection s as it can cause many dangerous pulmonary diseases (Krel et al., 2014).
The poultry sector in Egypt represents one of the most important agricultural industries that reaches more than 20 billion LE investments. Many Egyptian studies have been carried out on fungal infections especially aspergillosis in poultry and human beings (Refai et al., 2004; El-Tawab et al., 2015; Hassab et al., 2019). The results showed that this infection is widely distributed among chicken farms, hatcheries as well as workers in infected flocks (El-Bassiouni et al., 1988; Salem and Abdel Fatah, 2014; Mubarak and Mohamed, 2017; Radwan et al., 2019).
Accordingly, this review article gives a spotlight on the present situation of aspergillosis all over the world as well as in Egypt. The article focuses on the fungi, susceptibility, and transmission of infection, clinical picture in birds, human infection, diagnosis as well as prevention and control measures.
The causative agent
It has been demonstrated that Aspergillus species are worldwide distributed, normal inhabitant and ubiquitous fungal infections. These fungi present in the environment as soil saprophytes, but the spores multiply and disseminate in the organic matter under humid and poor ventilation conditions. Although the disease is prevailing throughout the year, its incidence is more frequently common in winter when indoor gas levels tend to be highest. Bad ventilation, increasing humidity and accumulation of organic matter in the litter are important environmental factors for the growth and multiplication of Aspergillus fungi (Zafra et al., 2008; Fang and Latgé, 2018).
A. fumigatus is the most common species of genus Aspergillus and family Trichocomaceae that was isolated from birds, animals and human (Talbot et al., 2018; Melo et al., 2019; Sabino et al., 2019). This species of fungi has many characters such as rapid growth, small size (1-4 µm), tolerance to a wide range of temperature (15°C to 70°C) and pH and low nutritional requirements (Lamoth, 2016; Fang and Latgé, 2018). Accordingly, these factors encourage the growth, multiplication and penetration of A. fumigatus into the lower respiratory tract of birds with the production of important toxic metabolites and virulence factors as protease, haemolysins, proteolytic enzymes, gliotoxin, abr1, alb1, arp1, aspHS and catA (Tekaia and Latgé, 2005; Rhodes, 2006; Hof and Kupfahl, 2009; Abad et al., 2010; Pena et al., 2010; Frisvad and LArsen, 2016). After a short time of inhalation of spores or conidia, the mucous covering the ciliated epithelial cells of the respiratory tract airways undergoe lysis followed by deep penetration of small conidia to the lower respiratory tract (Nganpiep and Maina, 2002).
Species as A. flavus, A. niger, A. glaucus and A. terreus have also been isolated from affected avian tissues, environment and human (Salem and Abdel Fatah, 2014). Other cryptic Aspergillus species as A. lentulus, A. udagawae, A. viridinutans, A. thermomutatus, A. novofumigatus and A. hiratsukae are representing 3 to 6% of Aspergillus infections worldwide. The low incidence of these Aspergillus species is related to their slow rate of production of mycotoxins, limited temperature tolerance and resistance to antifungal drugs (Alastruey-Izquierdo et al., 2014).
Susceptibility and transmission of infection
Almost all avian species are susceptible to Aspergillus infection due to poor vascularization of the air sacs as well as the presence of neutrophils with low efficacy against hyphae invasion (Tell, 2005; Xavier et al., 2011). Domestic (Sajid et al., 2006; Nawrot et al., 2019), wild (Xavier et al., 2007; Burco et al., 2012; Chege et al., 2013; Melo et al., 2019) and psittacine captive birds (McMillan and Petrak, 1989; Talbot et al., 2018) are susceptible to aspergillosis. Turkeys (Dyar et al., 1984; Richard et al., 1984; Singh et al., 2009; Olias et al., 2010; Lupo et al., 2010; Okwara, 2016; Hauck et al., 2020), chickens (Zafra et al., 2008; França et al., 2012), waterfowl (Okoye et al., 1989; Jordan et al., 2002; Beytut et al., 2004; Parker, 2011; Walker, 2012; Chung et al., 2020; Melo et al., 2020), pigeons (Beernaert et al., 2009), quails (Tell et al., 2009; Borah et al., 2010; Ahamad et al., 2018) and ratite species (Pérez et al., 2003; Yokota et al., 2004; Khosravi et al., 2008; Tijani et al., 2012; Araghi et al., 2014; Pazhanivel et al., 2018) showed this infection.
Aspergillus infections have been recorded in all ages of birds, but very young or very old birds are mostly affected (Coles, 1997; Akan et al., 2002). Small non-expanding bird’s lungs and distribution of air sacs facilitate colonization of the conidia in the caudal air sacs before they pass through the lungs in which gas exchange occurs (Nardoni et al., 2006).
Birds get infected with Aspergillus through inhalation of conidia or spores from polluted air and contaminated feed, soil, and droppings. Infection is commonly occurred s in the hatchery due to heavily contaminated environment “broader pneumonia”. Cracked eggs’ shell surfaces could be contaminated with Aspergillus spores and infect the embryos during the hatching process in the hatchery or during the brooding time (Oglesbee, 1997). Stressors like overcrowding, bad ventilation, high humidity of sawdust litter, warm temperature, malnutrition especially vitamin A deficiency as well as treatment with antibiotics increase the susceptibility to aspergillosis (Tell, 2005; Sultana et al., 2015).
Clinical picture in birds
The severity of clinical signs of aspergillosis in birds is mainly depends on the dose of the inhaled spores and the age and the immune status of the birds. Acute form may occur in the hatchery due to inhalation of a large dose of fungal spores and the affected chicks showed acute lower respiratory signs that manifested by gasping and difficult open-mouth breathing (dyspnea) (Aguilar and Redig, 1995). The morbidity rate in acute infection is high and can reach 90% within one to two days of Aspergillus infection. Recovered survived chicks may show off food, stunted growth, emaciation, eye swelling, or blindness.
Eye form of aspergillosis is represented as swollen eyelids with ocular discharge, cheesy yellowish exudates in the conjunctival sac, corneal opacity and blindness (Abrams et al., 2001; Lugauskas et al., 2004; Leishangthem et al., 2015). Experimental infection with A. fumigatus revealed ophthalmitis, retinitis, and iridocyclitis (Akan et al., 2002).
Finally, Aspergillus’s spores may be disseminated and metastasized in the brain causing nervous manifestations as torticollis (Throne Steinlage et al., 2003; Beernaert et al., 2010). Sometimes affected birds show subclinical infection without respiratory manifestations (Vegad, 2008).
Mycotic granulomatous dermatitis or cutaneous aspergillosis has been also reported in turkeys, chickens and pigeons along with pulmonary aspergillosis (Nardoni et al., 2006; Beernaert et al., 2010). Affected footpads showed keratinized epidermal disruption, encrustations and acute inflammation (Cannon, 1999; Hoppes et al., 2000; Stoute et al., 2009). Moreover, in a silky bantam chicken, A. fumigatus infection was associated with epidermal cysts in the comb (Curities, 1990; Suedmeyer et al., 2002). Death usually occurs due to pulmonary insufficiency, blindness, or brain affection.
Sub-acute form is usually developed in birds up to 2-weeks-old within 8-10 days of infection and the birds show less severe signs than acute form. Rattling, yellowish diarrhea and anemia could be seen (Richard, 1997; Atasever and Gümüssoy, 2004).
Chronic aspergillosis is usually occurring in adult breeders as sporadic and asymptomatic cases and it is associated with immunosuppression (Vanderheyden, 1993).
The high mortality rate was mostly recorded in young and severely affected chicks (Arné et al., 2011). Aspergillosis causes mortalities that vary from 4.5 up to 90% in the affected avian species (Beernaert et al., 2010; Da Silva Filho et al., 2015).
Post-mortem examination of dead birds revealed variable sizes whitish-yellow granulomatous foci or nodules in the syrinx, trachea, lungs and air sacs. Such granulomas could be disseminated in other organs as liver, heart, spleen, kidneys and intestine (Okoye et al., 1989; McMillan and Petrak, 1989; Jenkins, 1991; Singh et al., 2009). In addition, lung consolidation with single or multiple necrotic areas could be seen. Encephalitis and circumscribed white to greyish granulomas may also be noticed in the cerebellum and cerebrum with or without pulmonary affection and other lesions. Granulomatous osteoarthritis of the hip joints as well as femoral head necrosis were also observed in turkey poults (Olias et al., 2010). Pulmonary hypertension may lead to ventricular dilatation and ascites in chickens (Julian and Goryo, 1990; Suedmeyer et al., 2002; Cacciuttolo et al., 2009). Yellowish green mycelial hyphae could be observed in the affected organs (Cacciuttolo et al., 2009; Echenique et al., 2020).
Human affection
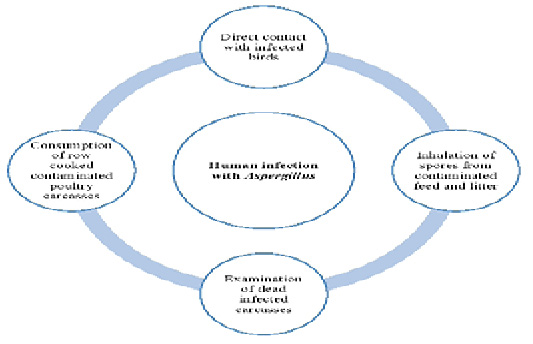
Humans can be infected with Aspergillus species in many ways (Figure 1). Aspergillus fungal species are transmitted to human s through handling with infected living birds, inhalation of spores from contaminated feed and litter, poor management and hygienic conditions, examination of dead infected carcasses as well as consumption of row cooked contaminated poultry carcasses. Furthermore, the role of wild birds in the transmission of Aspergillus species to humans can’t be ignored. Bird’s droppings containing conidia or infected dead birds can contaminate the environment with Aspergillus. So, human s can be infected through direct or indirect contact (Small and Nicholls, 2003).
It has been estimated that about 15 million people can be affected by aspergillosis with more than 1 million deaths annually (Global Action Fund for Fungal Infections, 2020). The most frequently found Aspergillus species in human cases is A. fumigatus (Escribano et al., 2013; Lamoth, 2016). Moreover, A. flavus is regarded as the second leading fungal cause of allergic and invasive infection in human in many countries (Chakrabarti et al., 2008; Pasqualotto, 2009; Hadrich et al., 2010, 2013). However, other species as A. lentulus, A. thermomutatus, A. pseudofischeri and A. felis have also been reported in the affected cases (Barrs et al., 2013; Howard, 2014; Negri et al., 2014).
Aspergillus infection is associated with several clinic spectrum like invasive aspergillosis, chronic pulmonary aspergillosis, Aspergillus bronchitis, and allergic bronchopulmonary aspergillosis (Kosmidis and Denning, 2015).
The severity of aspergillosis in humans depends mainly on the fungal extension in tissues and the immune response. After inhalation of conidia, macrophages in lungs’ alveoli detect, engulf and destroy them or severe allergy with systemic disorders may occur especially in immunocompromised humans (Brakhage et al., 2010; Milos et al., 2011). People with immunosuppressive disorders are highly susceptible to aspergillosis with a severe clinical picture and mortality rate (Maertens et al., 2001; Marr et al., 2002; Bassetti et al., 2015). Invasive aspergillosis (Seyedmousavi et al., 2015), chronic pulmonary aspergillosis (Denning et al., 2018), influenza-associated pulmonary aspergillosis in intensive care unit patients (Verweij et al., 2020) as well as COVID-associated pulmonary aspergillosis (Bartoletti et al., 2020; White et al., 2020) have been demonstrated. A recent study in hospitals of the United States showed an increase in the cases of invasive aspergillosis from 3 cases /10.000 people (in 1996) to 10 cases/10.000 (between 2009 and 2013) (Zilberberg et al., 2018).
Aspergillosis in Egypt
Some studies have been conducted in Egypt to demonstrate the situation of Aspergillus species infections in different governorates as well as some applied preventive measures. The incidence and distribution of aspergillosis among birds and human s in Egypt (Table 1). The results showed that this infection is widely distributed among chicken farms, hatcheries as well as workers handle with infected flocks (El-Bassiouni et al., 1988; Salem and Abdel Fatah, 2014; Mubarak and Mohamed, 2017; Radwan et al., 2019).
Diagnosis
Preliminary diagnosis of aspergillosis is usually depending on cumulative history, signs and lesions.
The history of bad environmental conditions (poor litter quality, bad ventilation and increased humidity), presence of severe respiratory signs or bird’s depletion may be indicative for aspergillosis. Clinical signs of aspergillosis are non-specific as they could be confused with other affections (Dahlhausen et al., 2004). The presence of white granulomatous nodules or cheesy plaques in the lungs, air sacs or other organs of affected birds may be diagnostic.
Definitive diagnosis of Aspergillus infection is based on cultural isolation of fungi or by microscopic examination (Jones and Orosz, 2000; Charlton et al., 2008; Beernaert et al., 2010).
Fungal hyphae could be seen after staining of affected organs with Periodic acid-Schiff, Bauer’s and Gridley’s, Grocott’s and Gomori as well as Methanamine Silver stains. In addition, wet smears from the specific nodules, fixed with 20% potassium hydroxide and stained with lactophenol cotton blue can be used for rapid detection of fungal hyphae. Swabs from sinuses or eyes can be subjected to wet mounts, fixed and stained with Parker’s India ink. Furthermore, histopathological examination of the affected organs revealed presence of granulomatous foci with central depressed coagulative necrosis surrounded by inflammatory cells as well as congestion of pulmonary and perialveolar blood vessels with perivascular edema (Girma et al., 2016).
Samples could be collected from the lesions (larynx, trachea, lungs, air sacs and brain) and cultured on selective specific media like Sabouraud’s dextrose agar or malt agar and incubated at 37°C for 24 hours. Aspergillus species can be identified by detection of the characteristic conidial head and colony (Aguilar and Redig, 1995; Dahlhausen et al., 2004). It should be noted that negative culture cannot indicate absence of Aspergillus infection (Redig, 1994).
Biochemical and hematological parameters are also diagnostic indicators for Aspergillus infection (Jones and Orosz, 2000).
Serological tests like enzyme-linked immunosorbent assay (Brown and Redig, 1994; Redig et al., 1997; Le Loch et al., 2005; Arca-Ruibal et al., 2006), immunohistochemistry
Table 1: Incidence and distribution of aspergillosis among birds and human in Egypt.
| Findings | References |
|
The author studied the epidemiology of Aspergillus species and demonstrated the presence of fungi un in slaughtered and frozen chickens’ meat. |
|
|
The authors proved the zoonotic nature of Aspergillus species as they have been isolated from chickens and related human workers. |
|
|
The authors studied the pathogenicity and enzymatic activities of A. fumigatus, A. flavus and A. niger isolated from chickens and their environment (feed, litter, water and air). |
|
|
Lungs of 100 diseased chickens, 80 samples of litter, feed, water and air (20; each) as well as 30 sputum and 30 serum samples were collected from diseased contact human in Kalyoubia governorate. The results demonstrated presence of Aspergillus species in percentages 24, 55, 55, 10, 50 and 23.3% in lungs, feed, litter, water, air and sputum, respectively. A. fumigatus was the predominant fungus that isolated from all samples followed by A. niger and A. flavus. The incidence of infection was high in hot and humid seasons and as well as in chickens reared on saw dust litter as compared to rice husk litter. Aspergilli were isolated from all age groups of human but the majority of infection were among the 20-40 years group (26.7%), both males (23.5%) and females (23.1%) are equal in susceptibility to infection but higher in farm workers (29.4%) than in farm owners and Veterinarians. Moreover, A. fumigatus was present in sputum (13.3%) and but in serum (10%) after using ELISA IgG. |
|
|
Samples including lung, air sac, crop, liver and brain were collected from 225, 1- 30 days old morbid and dead chickens for detection of mycotic pathogens in Dakahlia governorate. The results revealed isolation of fungi from 503 samples (44.7%); represented as 119 positive samples (10.6%) from diseased chickens and 384 positive samples (34.1 %) from dead birds. Moulds and yeasts were detected from 651 out of 1125 samples, where 219 (33.6%) were isolated from lung, 199 (30.6%), 119 (18.3%), 91 (14.0%) and 23 (3.5%) from air sac, liver, crop and brain, respectively. Moreover, A. fumigatus was the most predominant isolate 141 (21.7%) followed by A. flavus 126 (19.4%), A. niger 111 (17.1%), A. terreus 20 (3.1%) and A. ochraceus 4 (0.6%). Moreover, A. fumigatus A. flavus and A. niger were able to amplify the ITS–5.8S rDNA region providing a single PCR product of about 600 bp. |
|
|
The author showed isolation of Aspergillus species from dead-in-shell eggs in incidence rate of (66.9%) as A. fumigatus and A. niger were the major isolated fungi. |
|
|
In this study, most of the fungal species especially A. niger, and A. flavus were isolated from sputum of poultry-men working in such five farm in Mounofia governorate. In addition, Aspergillus species was detected in the blood serum samples by using ELISA IgG. |
|
|
This study demonstrated the incidence of Aspergillus species in chicks and farm workers in Qena governorate. It has been found that 29 out of 40 chick’s laryngeal swabs (72.5%) and 15 out of 40 farm worker’s sputum samples (37.5%) were positive for Aspergillus species. The incidence of A. flavus, A. fumigatus and A. niger were 60, 10 and 30% of chicks samples, however, 12.5, 15 and 30% of workers samples were positive for these species, respectively. Moreover, A. ochraceus was also detected (5%). From both chicks and workers, aspHS gene was detected in 75 and 33.3% of A. fumigatus strains, respectively. |
|
|
The authors found high prevalence rate of fungal isolates in tracheal and cloacal swabs (39.3 - 48.1%) of broiler breeder chickens as well as in feed and water samples (37.5% and 28.6%), respectively. A. niger, A. flavus, and A. terreus were the predominantly fungi. The ß-Tubulin genes PCR-RFLP of selected Aspergillus isolates showed a characteristic restriction pattern for each species. The β-tubulin gene phylogenetic and sequence analysis of selected A. flavus, and A. terreus from breeder chickens and their hatching chicks indicated their relatedness to isolates from bronchopulmonary aspergillosis in humans in the Middle East. |
|
|
They found an antifungal activity of essential oils of oregano, thyme, rosemary and clove against some fungi especially food-borne fungal species. |
|
|
A total of 225 samples were collected from 75 human swabs and samples (40 ear, 29 vagina and 6 sputum) and 150 from broiler chickens for fungal examination in El-Fayoum and Beni-Suef governorates. About 129 fungal isolates were isolated and represented 22 isolates (29.3%) from human [15 (37.5%) A. fumigatus isolates in ear and 7 (24.1%) A. fumigatus isolates in vagina], no A. fumigatus was recovered from sputum, while 53 (35.3%) A. fumigatus isolates was recovered from broiler chicken. Moreover, thymol and carvacrol oils completely inhibited the growth of different fungal isolates at concentrations of 1% and 0.1%. However, 0.01% concentration of these oils was too weak to inhibit the fungal growth, but it completely faint the colonial colour and converted it into white arial mycelium. The PCR assay amplified 250 bp fragment of A. fumigatus and A. niger and the sequence of them was performed. |
|
|
They collected laryngeal swabs from morbid chickens, ducks, and pigeons, (30 from each), soil, water, and air (30 from each), and 30 human’s laryngeal swabs from those in contact with diseased birds as well as 30 sputum samples from patients admitted to Qena Chest Hospital with pulmonary complaints. The results showed presence of Aspergillus in ducks and pigeons with incidences of 80 and 56.6%, respectively. These incidences were 66.6, 40 and 100% in soil, water, and air, respectively. Phenotypic characterization of Aspergillus species displayed presence of A. fumigatus, A. flavus, and A. niger. These species were also detected in 36.3% of human laryngeal swabs and 50% of sputum samples. Moreover, they molecularly identified Aspergillus strains using ITS region sequences. |
|
| The authors demonstrated that cinnamon, thyme, and anise essential oils have strong antifungal activity. In addition, they confirmed that ITS region sequences is a good marker for phylogenetic analysis of most fungi. |
(Carrasco et al., 1993; Jensen et al., 1997; Beytut et al., 2004; Beytut, 2007), galactomannan assay and plasma protein electrophoresis (Cray et al., 2006, 2009a, b) as well as using of monoclonal or polyclonal antibodies are confirmatory for aspergillosis.
Lateral and ventrodorsal radio-graphical views of suspected birds can indicate of aspergillosis (Jones and Orosz, 2000). Endoscopically, yellowish-white plaques covered with green or gray hyphae of fungal growth could be observed in the abdominal air sacs of Aspergillus infected birds (Taylor, 1993; Oglesbee, 1997).
Recent molecular techniques like real time polymerase chain reaction (PCR), nucleic acid sequencing based amplification and molecular beacon technology are now available for diagnosis of Aspergillus infection (Dahlhausen et al., 2004; Balajee et al., 2009; Saleemi et al., 2012; Zhao and Perlin, 2013). A phylogenetic analysis of Aspergillus strains based Internal Transcribed Spacer (ITS) gene-based PCR was conducted in Egypt for broiler breeder chickens, dead-in-shell and hatched chicks (Radwan et al., 2018a), for the environment and hospitalized patient (Hassab et al., 2019), and for diseased broiler chickens (Abed et al., 2021).
Prevention and control
There is no effective treatment for fungal infected poultry, and therefore, the only effective way to protect chickens is prevention (Arné et al., 2011). Setters, brooders, hatcheries as well as poultry houses should be put under strict hygienic and sanitary conditions (Chute and Richard, 1991; Beernaert et al., 2010). Thorough cleaning, disinfection and fumigation using formaldehyde or antifungal compounds as thiabendazole (120-360 g/m3) is recommended (Pattisson et al., 2008). Decontamination of bedding and environmental disinfection using azoles is common (Nawrot et al., 2019). Cracked dirty eggs should be removed and not used for the hatching process. Controlling the environmental conditions through avoidance of moldy litter or feed is a must. The litter should be kept dry and the moldy one should be immediately removed and replaced by a clean and new one. Sometimes moldy litter could be treated with antifungal preparations as copper sulphate or nystatin (Dyar et al., 1984). Feeders and drinkers should be thoroughly cleaned and disinfected (Kunkle, 2003). A trial for preparation of vaccine was developed (Richard et al., 1991), but no vaccine is commercially available.
Morbid birds should be separated from the flock. Treatment is of low value in case of severe aspergillosis with extensive lesions and poor prognosis. In such cases, antifungal drugs cannot effect on the fungus that surrounded by inflammatory reactions. Treatment is recommended and fruitful only in mild and early stages of aspergillosis using specific and supportive treatments. Copper sulphate could be used in the feed for 6 days treatment or in the drinking water at a concentration of 1:2000. In addition, one or more systemic antifungal drugs are used. The commonlay used antifungal drugs include itraconazole, miconazole, eniconazole, clotrimazole, ketoconazole, fluconazole, Amphotercin B and fungicidin (Dhama et al., 2013).
It has been demonstrated that there is an increase in the resistance rate to the classical antifungal drugs, besides, most of these drugs are only fungistatic. Therefore, new trends have been now applied to counteract such infections. Essential oils of some plants (phytobiotics) showed broad spectrum potential antifungal activities in poultry (Pinto et al., 2009; Radwan et al., 2018b; Abed et al., 2021).
Different studies have been done in vitro to demonstrate the antifungal activity of plant’s essential oils against different fungal growth (Chuang et al., 2007; Kędzia and Hołderna-Kędzia, 2007; Yang and Clausen, 2007; Pinto et al., 2009). A mixture of cinnamon, lavender, rosemary, and sage oils at 1% concentration completely inhibited the growth of Aspergillus species spores (Cvek et al., 2010). However, cinnamon or cinnamon fortified with cinnamaldehyde essential oils exhibited strong suppressive activity against Aspergillus species at low concentration of 0.1% (Abed et al., 2021), or a concentration of 4% (López et al., 2005). The antifungal activity of thyme essential oil and thymol against molds was also studied (Klarić et al., 2007). The results showed that the vaporous phase of thyme oil showed long-lasting antifungal activity on molds from damp dwellings. In this context, Witkowska et al. (2016) found that in the last two weeks of broilers rearing, Aspergillus species counts were 75% (thyme oil) and 46% (peppermint oil) lower in comparison with the control group.
To avoid occupational Aspergillus infection in human, it is recommended to take special precautions as wearing gloves and masks during handling of birds, avoidance of damp litter and dust by adequate ventilation of poultry farms and moldy feed shouldn’t be given to birds (Mubarak and Mohamed, 2017). Besides, once Aspergillus infection has been detected on the farm, a rapid molecular diagnosis, treatment of infected patients and hygienic disposal of dead birds are very important to reduce the spreading of infection (Mubarak and Mohamed, 2017).
CONCLUSIONS AND RECOMMENDATIONS
Aspergillosis is regarded a very important fungal disease affecting birds with severe losses in poultry production system as well as its zoonotic importance. Therefore, more and periodical research work should be carried out to investigate the aspergillosis situation as well as detection of new methods for disease prevention.
NOVELTY STATEMENT
Avian aspergillosis along with its hazardous effect on the human’s health all over the world and especially in Egypt is an issue with a great significance and concern.
Conflict of interest
The author declares no conflict of interest.
REFERENCES