Advances in Animal and Veterinary Sciences
Research Article
Antiviral Activity of Curcuma Longa and Bottle Gourd Extracts Against Field and Vaccine Strains of Marek’s Disease Virus
Samar S. Ewies1*, Mahmoud Samir2, Abdelsatar A. Arafa2, Sabry M. Tamam1, Hanafy M. Madbouly1
1Virology Department Faculty of Veterinary Medicine, Beni-Suef University, Egypt; 2Reference Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute, Agriculture Research Center, Giza, Egypt.
Abstract | In the present study, the antiviral activity of Curcumin (Curcuma longa) and bottle gourd (Lagenaria siceraria) extracts was evaluated against the field and vaccine strains of Marek’s disease virus (MDV). Plaque reduction assays were performed and the obtained results showed that both Curcumin and bottle gourd had a reduction effect on the number of plaques in comparison with the control vaccines. Curcumin exhibited prominent antiviral effect expressed as 80% and 66% plaque reduction percent (PR%) against HVT and Rispen’s respectively at the 1:8 virus dilution. While, bottle gourd has moderate antiviral action at 1:4 dilution; it has 40% and 33% PR% against HVT and Rispen’s respectively at dilution 1:8. The obtained results were evaluated using polymerase chain reaction PCR SYBR Mix and showed antiviral activity of these plants against Marek’s disease virus as Curcumin 26.1±1.7a, Bottle gourd 25.62 ±1.1a, Positive control 17.125 ±0.6b; these results indicate that both Curcumin and bottle gourd have an antiviral effect against recently isolated wild type MDV.
Keywords | Marek’s disease virus, Curcuma longa, Bottle gourd, Polymerase chain reaction SYBR green mix, Plaque reduction assay.
Received | March 06, 2021; Accepted | March 31, 2021; Published | August 15, 2021
*Correspondence | Samar S. Ewies, Virology Department Faculty of Veterinary Medicine, Beni-Suef University, Egypt; Email: Samarewies@yahoo.co.uk
Citation | Ewies SS, Samir M, Arafa AA, Tamam SM, Madbouly HM (2021). Antiviral activity of curcuma longa and bottle gourd extracts against field and vaccine strains of marek’s disease virus. Adv. Anim. Vet. Sci. 9(10): 1525-1531.
DOI | https://dx.doi.org/10.17582/journal.aavs/2021/9.10.1525.1531
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Ewies et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Herbal preparations have been used as therapeutics for treatment of various illnesses and are reported to be safer and less toxic than synthetic drugs (Dubey et al., 2004). A polyherbal formulated drug, has been used for immunoprophylactic and immunotherapeutic capabilities and no toxic effects were found (Sulaiman et al., 2010). Many in vitro and in vivo studies from different parts of the world have been conducted in the last few years to evaluate the antiviral activity of medicinal plants (Tamam and El-Yamany 2008). Hundreds of plant and herbal species have the potential as antiviral agents. A wide variety of active phytochemicals, including the flavonoids, terpenoids, sulphides, saponins, lignans, polyphenolics, furyl compounds, coumarins, alkaloids, thipphenes, polines, proteins and peptides. In addition, some volatile essential oils have been identified which exhibited a high level of antiviral activity (Fadwa, 2009).
The relative success achieved recently using herbal extracts of various species, which capable of acting therapeutically during various viral infections increasing the future for using phyto antiviral agents. Curcumin (Curcuma longa) or Turmeric belongs to the family of ginger (Zingiberaceae) and natively grows in India and Southeast Asia (Praditya et al., 2019). In vitro studies revealed that Curcumin has antiviral effect on different avian influenza viruses (AIVs) as it inhabit the virus uptake, replication and release (Han et al., 2018). Meanwhile, other studies reported that Curcumin has antiviral activity against ARBO viruses (Tongaviridae) as it prevent the entry by preventing the virus binding to host cells (Mounce et al., 2017). In addition, Curcumin treatment of infected cells with ARBO viruses resulted in the intracellular accumulation of viral proteins and reduction of viral particles production (Padilla et al., 2014). The antiviral effect of Curcumin against respiratory syncytial virus (RSV) infections has been identified due to its effect on RSV replication, budding from human nasal epithelial cells and increased the epithelial barrier function (Obata et al., (2013).
Calabash (Lagenaria siceraria) of the family Cucurbitaceae, also known as bottle gourd, is grown in its native areas of Africa and the Americas and in tropical countries, such as India, Japan and Thailand (Shah et al., 2010). Irshad et al. (2010) reported that the fruit of Gourd contain flavonoids, alkaloids, cucurbitacins, steroids and saponins, Also, Duke (1999) mentioned that it contain triterpenoids and C-flavone glycosides (Deshpande et al., 2007) stated that it contain ellagitannins, (Sonja and Hermann, 2000) mentioned that it contain triterpenoid cucurbitacins B, D, G, H, 22-deoxy cucurbitacin and (Shirwaikar and Sreenivasan, 1996) said that it contain fucosterol and campesterol. Some experimental results revealed that L.Siceraria possess significant anticancer activity which may be due to its cytotoxicity and anti-oxidant properties (Saha et al., 2011). An ethanolic extract of L. siceraria fruit contains antioxidant compounds equivalent to 50 mg·kg−1 of vitamin C plus 50 mg·kg−1 of vitamin E (Deshpande et al., 2008).
The aim of the present study is to study the antiviral activity of Curcumin and Bottle gourd (Lagenaria siceraria); extracts against field and vaccine strains of Marek’s disease virus (MDV).
MATERIALS and METHOD
Viruses and Cells
Herpes Virus of turkey “HVT” vaccine containing live Marek’s disease virus serotype 3 and the Rispen’s vaccine containing live Marek’s disease virus serotype 1 strain CVI 988 (MERIAL, INC, USA) were used. The wild type Marek’s disease virus strain MW194840 Eg-F1352-S4/2020 was used as field strain.
Duck embryo fibroblast (DEF) primary cell culture was prepared from duck embryos at 12-14 days old embryo following the standard procedures (Freshney, 2000). Cells were used for cytotoxicity experiments for Curcumin and bottle gourd, virus titration and antiviral studies. Cells were grown in minimum essential media (MEM) supplemented with 10% heat inactivated fetal calf serum (FCS).
Plants and Ethanolic Extraction of Bottle gourd
The Curcumin was purchased from Sigma® (Cat Number 458-37-7, Sigma Aldrich, Germany), while the Bottle gourd plant was purchased from a local market in Egypt. The fruit of the plant was dried in hot air oven, ground mechanically until it became small fragments and then 2 grams was taken and soaked in 10mL of 70% ethanol. The mixture was kept for five days then coarse filtration to the mixture was conducted through Whatman® filter paper and the filtrate was dried at room temperature (Ghule et al., 2006).
Viruses Titration and Cytotoxicity Experiments
Viruses titration were carried out as previously described (Hitcher and white, 1958) and the plaque forming unit (PFU) were calculated (OIE, 2018).
Cytotoxicity of different doses for Curcumin (5 µg, 10 µg, 20 µg and 50 µg) or bottle gourd extract (5 µL, 10 µL, 20 µL and 50 µL) were determined based on the microscopic examination of DEF cells for the cytopathic effect (CPE). Cells were grown in 96 well tissue culture microtiter plate; every dose was inoculated in duplicate either the bottle gourd extract or Curcumin. The plate was incubated at 370C in CO2 incubator with daily microscopic examination under inverted microscope for 3 successive days for CPE detection.
Plaque Reduction antiviral assay of bottle gourd and Curcumin
The antiviral activity of bottle gourd extract against MDV was determined quantitatively based on microscopic examination (CPE) and virus quantification using real time Sybrgreen PCR assay. Cells were grown in 24 well plate, cells were infected with HVT (6480 Pfu/200 µL) or Rispen’s (5200 pfu/200 µL) mixed with 20uL bottle gourd extract or 20µg Curcumin in duplicate compared to negative control cells. The plate was incubated at 370C in 5% CO2 incubator with daily microscopic examination under inverted microscope for 3 successive days for cytopathic effect (CPE) detection.
Antiviral activity of Curcumin and bottle gourd by Sybrgreen PCR in culture cells
Cells were grown in 24-well plate and infected with filed virus (Eg-F1352-S4/2020) of MDV (400 pfu/200 µL) mixed with 20 µL bottle gourd extract or 20ug Curcumin powder in duplicate wells. The plate was incubated at 370C in 5% CO2 incubator with daily microscopic examination under inverted microscope for 3 successive days for CPE detection compared to negative control cells.
The PCR was performed using 2X ABT SYBR mix (Invitrogen, CA, USA) using set of primers F-3’ GGATCGCCCACCACGATTACTACC 5’ and R- 3’ACTGCCTCACACAACCTCATCTCC 5’ (Handberg et al., 2001). The cycling profile was as follow; an initial denaturation step at 95 ᵒC for 3 min, 40 cycles of PCR amplification at 95 ᵒC for 15 sec, 52 ᵒC for 30 sec and 72 ᵒC for 30 sec. The obtained raw data was exported to Microsoft Excel then prepared for statistical analysis and the amplified fragments were separated by gel electrophoresis using 1.5% agarose gel stained with ethidium bromide and visualized under ultraviolet light.
Statistical Analysis
Statistical analysis was performed using SPSS v.25. Results were expressed as mean ± standard deviation and all statistical comparisons were made by Duncan’s test post hoc analysis. Values of p<0.05 were considered significant while those of p>0.05 were considered non-significant.
Results and Discussion
Many traditional medicinal plants have strong antiviral activity; some of them have already been used to treat animals and humans suffering from viral infection.
The antiviral activity of Curcumin against different viruses has been reported due to its action against avian influenza virus (Han et al., 2018) and ARBO viruses (Mounce et al., 2017; Praditya et al 2019). The bottle gourd possesses a significant anticancer activity which may be due to its cytotoxicity and antioxidant properties (Saha et al., 2011).
Cytotoxicity of ethanolic extract of bottle gourd in culture cells (Table 1) was examined through four doses (5µl, 10µl, 20µl and 50µl) of ethyl extract of tested bottle gourd then evaluated in cultured cells (DEFs). The negative results indicated the dilutions that were not toxic to cells but positive result (+) indicated the highest dilution (50ul) was toxic to the cultured cells (DEFs). Depending on these results, the dilution 20 ul was used as the ideal dilution in antiviral assay. The same method was used for Cytotoxicity of Curcumin in culture cells, where 100 ml of the media containing four doses of Curcumin (5µg, 10µg, 20µg and 50µg) were evaluated in cultured cells (DEFs). The highest dilution (50µg) was toxic to the cultured cells (DEFs). So we used 20µg as the ideal dilution in antiviral assay. The results of cytotoxicity showed clearly that the doses (5, 10 and 20 µl/wells for Curcumin and 5, 10 and 20 µg/wells for bottle gourd) were not toxic to the cells for 3 days while the high dose (50µl) was toxic to culture cells resulting in cell lyses and detachment from surface of micro titer plate after 24 - 48 hrs post inoculation. Therefore, we used a dose of 20ul/well for antiviral experiments (Table 1).
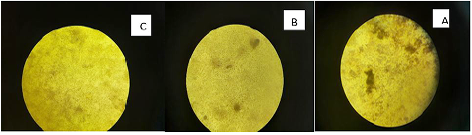
Marek’s disease virus (MDV) is double stranded DNA virus in which capsid is assembled in the nucleus and enveloped by budding through the nuclear membrane. The current study was planned to evaluate the antiviral activity of Curcumin and bottle gourd extracts against MDV. Plaque reduction assay were performed, and the obtained results showed that both Curcumin and bottle gourd had reduction effect on the number of plaques in comparison with the control vaccines as shown in (Table 2) and in (Figure 1) However, Curcumin had the most prominent plaque reduction effect on both HVT and Respin’s vaccines.
Table 1: Cytotoxicity of Bottle Gourd and Curcumin in Culture Cells.
| Plant | Cytopathic effect (CPE) | |||
| 5 | 10 | 20 | 50 | |
| Bottle gourd (µl) | - | - | - | + |
| Curcumin (ug) | - | - | - | + |
Result of cytotoxicity of ethanolic extract of bottle gourd to culture cells as Four doses (5µl, 10µl, 20µl and 50µl) of ethyl extract of tested bottle gourd and Curcumin to culture cells as 100ml of the media containing the different Four doses of Curcumin (5µg, 10µg, 20µg and 50µg) were evaluated in cultured cells (DEFs) (-) mean that the lowest dilution was not toxic to culture cells but (+) mean that the highest dilution 50ul was toxic to the cultured cells (DEFs) So, 20ul was used as the ideal dilution for bottle gourd, the highest dilution 50µg was toxic to the cultured cells (DEFs) So, 20µg was used as the ideal dilution for Curcumin.
Curcumin exhibited prominent antiviral effect expressed as 52% plaque reduction percent (PR%) against HVT and 48% against Rispen’s at the virus original concentration. Then increased up to 80% and 66% in the 1:8 dilution. While, bottle gourd has no significant effect at high virus concentration (original) then started to exhibit moderate antiviral action in 1:2 and 1:4 dilutions. It has 40% and 33% PR against HVT and Rispen’s respectively at dilution 1:8. Dilutions after 1:8 were not statistically approved or there was no virus at these dilutions (Table 2).
Due to the inability of filed virus (Eg-F1352-S4/2020) to induce CPE in cultured cells within few serial passages, so we made real-time Sybrgreen PCR in cultured cells after the third passage to detect virus particles and to estimate the reduction of virus after treatment with plant extracts. Data are expressed as mean ± standard deviation.
Table 2: The Plaque Reduction Assay for Both Curcumin and Bottle Gourd in Comparison with the Positive Controls.
|
Extract |
Bottle gourd |
Curcumin |
Positive Control |
|||
|
Virus dilution |
HVT PFU/ml |
Rispen’s PFU/ml |
HVT PFU/ml |
Rispen’s PFU/ml |
HVT PFU/ml |
Rispen’s PFU/ml |
|
Original Virus PFU (PR%) |
145A (7%) |
120B (4%) |
75a (52%) |
65b (48%) |
155A |
125B |
|
1:2 |
38A (5%) |
28B (15%) |
20a (50%) |
15b (55%) |
40A |
33B |
|
1:4 |
9A (10%) |
8B (11%) |
6a (40%) |
3b (66%) |
10A |
9B |
|
1:8 |
2a (40%) |
2b (33%) |
1a (80%) |
1b (66%) |
5A |
3B |
|
1:16 |
1 |
0 |
0 |
0 |
1 |
1 |
|
1:32 |
0 |
0 |
0 |
0 |
0 |
0 |
|
1:64 |
0 |
0 |
0 |
0 |
0 |
0 |
|
1:128 |
0 |
0 |
0 |
0 |
0 |
0 |
PFU = plaque forming unit. PR%= Plaque reduction percent in comparison to corresponding positive control. Same capital letters superscript indicate no significant difference, small letters superscript indicate significant difference. A: comparison with HVT, B: comparison with Rispen’s MDV.
Table 3: Antiviral Activity of Curcumin and Bottle Gourd by Sybrgreen PCR in Culture Cells.
| Mean ± standard deviation | Group |
|
26.1±1.7a |
Curcumin |
|
25.62 ±1.1a |
Bottle gourd |
|
17.125 ±0.6b |
Positive control |
The antiviral effect of Curcumin and bottle gourd in comparison with the positive control.

Figure 2: Illustrate the Dissociation Curve of MDV with Curcumin in Comparison with Control Positive Virus.
Number of detected samples in each group is four for each parameter; means which have the same superscript symbol (s) are not significantly different shown in (Table 3). The result by dissociation curve were evaluated and indicated that both Curcumin and bottle gourd have antiviral effect against MDV and this result agree with the result of Ct of the virus with both Curcumin and bottle gourd in comparison with the control positive as shown in (Figure 2) and (Figure 3) and the result of chart point as shown in (Figure 4) and the results of gel electrophoresis to the produced PCR products for confirmation of results for the specific band at 318 bp (Figure 5).
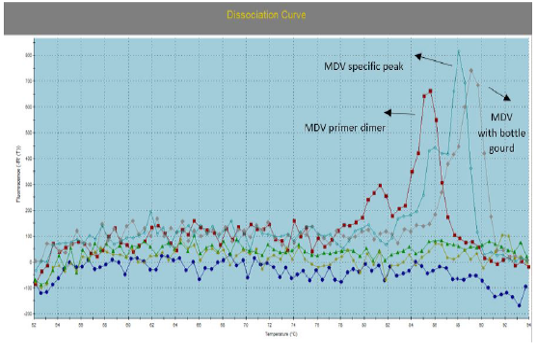
Figure 3: Illustrate the Dissociation Curve of MDV with Bottle Gourd in Comparison with Control Positive Virus.
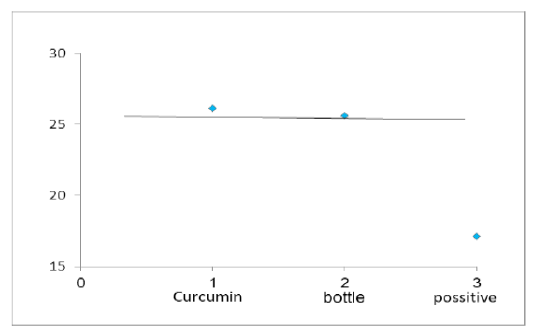
Figure 4: Chart Point Indicate that Both Curcumin and Bottle Gourd have Antiviral Activity against MDV in Comparison with the Control Positive Virus.
For evaluation of antiviral activity of bottle gourd and Curcumin, Marek’s disease virus was used as a model, which is an oncogenic poultry herpes virus, causing not only death of chickens directly, but also immunosuppression of infected chickens Plaque assay was performed using both HVT and Rispen as illustrated in Table 2 as the number of plaques were calculated (PFU) per ml in which the vaccines used alone as controls and each vaccine was used with bottle gourd and HVT each alone and from the result we found that both Curcumin and bottle gourd had reduction effect on the number of plaques. Data presented in Table 3 showed clearly that both Curcumin and bottle gourd have antiviral effect on MDV as they decreased the virus titers in infected cells in comparison with the positive control virus only. The result by dissociation curve were evaluated and indicated that both Curcumin and bottle gourd have antiviral effect against MDV and this result agree with the real time PCR assay for both Curcumin and bottle gourd in comparison with the virus control.
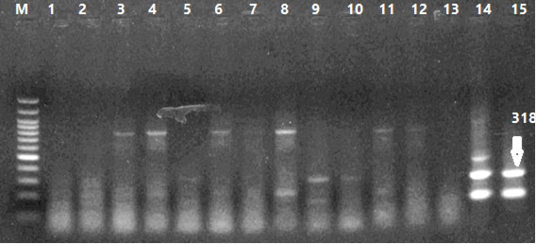
Figure 5: Results of PCR Products Gel Electrophoresis. 1-4 Curcumin Dilutions, 5-8 Bottle Gourd Dilutions, 9-12 Virus Dilutions, 13 Negative Control, 14 Positive Virus Control (HVT), 15 Positive Virus Control (wild type: Eg-F1352-S4/2020).
In previous work, it could be proposed that bottle gourd is exhibiting its protective effect by protecting against DNA damage. The antiviral activity of Bottle gourd may be due to it possesses ascorbic acid which prevents DNA damage in skin (Placzek et al., 2005). In addition to the presence of Luteolin that can also protect against DNA damage (W¨olfle et al., 2011). These findings indicate the protective role of derivatives present in bottle gourd. Furthermore, the preventive effects of various phytochemicals have been reported against DNA damage (Nigam et al., 2007).
Chang et al. 2013 used plaque reduction assay to investigate the antiviral activity of Zingiber officinale against human respiratory syncytial virus by in both human upper (HEp-2) and lower (A549) respiratory tract cell lines. Another work has been done on black seed oil from Nigella sativa to study its antiviral effect using murine cytomegalovirus as a model. The results showed a remarkable antiviral effect of black seed oil against cytomegalovirus infection that may be mediated by initiating the innate immunity (Salem et al., 2000).
The essential oils displayed strong antiviral effects against some viruses like Herpes simplex virus (HSV) as examined in previous study the antiviral activity of the essential oil of fruit sample of Foeniculum vulgare against the DNA HSV type-1. The extracted oil inhibited the virus significantly by a maximum cytopathic inhibitory concentration ranged between 0.8 and 0.025 μg/mL.
In addition, plant extracts exhibit efficient antioxidant properties due to their phyto-constituents, including phenolics (Jitoe et al., 1992; Lee and Hwang, 2003).
One of potent natural antiviral agents is Curcumin, a major antioxidant compound and a main constituent of the spice turmeric (Curcuma longa) as it inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of P300/CBP histone acetyltransferase activity and have remarkable antiviral effects on HSV-1 in cell culture. (Kutluay et al., 2008).
CONCLUSIONS
In conclusion, both Curcumin and bottle gourd herbal extracts have an important antiviral activity against MDV on cell culture. Curcumin exhibited much prominent effect than bottle gourd on cell culture against wild type MDV than bottle gourd. It is recommended to study their fractionation properties to identify their active constituents. Further studies are recommended to evaluate their effect on chickens infected with MDV to reduce its impact on chicken loss or decrease in their performance.
AUTHORS CONTRIBUTIONS
Hanafy M Madbouly conceived of the presented idea and planned the experiments. Samar S Ewies and Mahmoud samir carried out the experiment, and wrote the manuscript with input and support from all authors. Sabry M Tamam designed the model and the computational framework and analysed the data. Abdelsatar arafa worked out almost all of the technical details, and performed the numerical calculations for the suggested experiment. All authors discussed the results, provided critical feedback and helped shape the research and contributed to the final.
ACKNOWLEDGEMENT
The authors would like to thank the staff members of Reference Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute, Egypt for offering facilities in processing the materials used in this study.
Conflict of interest
Authors declared no conflict of interests exist.
References