Advances in Animal and Veterinary Sciences
Research Article
In Vitro Potency of a Crude Aqueous Extract of Artocarpus heterophyllus Leaves as an Anthelmintic against Haemonchus contortus in Jawarandu Goats
Budi Purwo Widiarso1*, Dias Aprita Dewi1, Kurniati Sarwendah2, Dhestalia Eka Pratiwi3
1Department of Animal Production Technology, Politeknik Pembangunan Pertanian Yogyakarta-Magelang, Indonesia Desa Purwosari Tegalrejo Magelang, 56192; 2The assesment Institute for Foods, Drug, and cosmetics , Indonesian Council of Ulama, Yogyakarta,Indonesia.Jalan kapas I /1 Semaki Kota Yogyakarta; 3Department of Agriculture Extension and Animal welfare, Politeknik Pembangunan Pertanian Yogyakarta-Magelang, Indonesia. Desa Purwosari Tegalrejo Magelang, 56192.
Abstract | Haemonchosis is a parasitic disease that often occurs in goats in Indonesia. In sub-tropical and tropical areas, the mortality, decreased production, stunted growth, and low weight gain due to haemonchosis have substantial economic impacts. Jackfruit (Artocarpus heterophyllus) is recognized as a plant that has the potential for anthelmintic activity because its leaves contain tannins. The aim of this study was to determine the in vitro effects of a crude aqueous extract of jackfruit leaves on the parasitic worm Haemonchus contortus. Six groups of 10 adult worms were immersed in different doses (0%, 0.25%, 0.5%, 0.75%, and 1%) of a jackfruit leaf extract or in albendazole (positive control) and assessed five times or as five replications every hour for up to 4 hours. Two-way ANOVA was used to analyze worm mortality rates at the various treatment doses and observation times, while one way ANOVA was used to analyze the differences in worm morphometry. The jackfruit extract at various doses and observation times had a significant effect on worm mortality and altered the worm morphometry, especially the male and female body length, cervical papilla width, spicula length in males, and vulva flap length in females. A 1% dose of jackfruit leaf extract caused the greatest worm mortality. The in vitro worm mortality and morphometry examinations support the use of Artocarpus heterophyllus aqueous crude leaf extracts as potential anthelmintics against Haemonchus contortus.
Keywords | Artocarpus heterophyllus, Haemonchus contortus,Goat, anthelmintic, in vitro
Received | May 14, 2021; Accepted | June 12, 2021; Published | July 28, 2021
*Correspondence | Budi Purwo Widiarso, Department of Animal Production Technology, Politeknik Pembangunan Pertanian Yogyakarta-Magelang, Indonesia Desa Purwosari Tegalrejo Magelang, 56192; Email: budipw2000@yahoo.com
Citation | Widiarso BP, Dewi DA, Sarwendah K, Pratiwi DE (2021). In vitro potency of a crude aqueous extract of artocarpus heterophyllus leaves as an anthelmintic against haemonchus contortus in jawarandu goats. Adv. Anim. Vet. Sci. 9(9): 1498-1503.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1498.1503
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Widiarso et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Health aspects need to be considered in raising livestock like goats. One of the influencing factors is the presence of parasites that cause disease (Dhewiyanti et al., 2015). Parasitic diseases caused by gastrointestinal nematodes that cause gastrointestinal infections are particularly harmful to goats. Decreases in livestock production, such as weight loss and impaired growth, are key impacts of nematode worm infections, along with decreased immunity to disease, especially in young ruminants. The types of nematode worms that often attack goats are Haemonchus contortus, Trichostrongylus spp., and Oesophagostomum spp.
H. contortus is responsible for the disease known as haemonchosis, a parasitic disease that often occurs in goats in Indonesia. H. contortus often attacks and develops in the digestive tract, especially in the abomasum (Beriajaya and Haryuningtyas, 2002). In sub-tropical and tropical areas, haemonchosis causes mortality, decreased production, stunted growth, and low weight gain as its greatest economic impacts (Mengist et al., 2014). Animals that are grazing will eat the larvae of H. contortus that are attached to the grass. The larvae then enter the digestive tract and develop in the abomasum. However, this type of worm infestation can be prevented by natural anthelmintics derived from feed plants. In addition to being inexpensive, these natural compounds also have no adverse effects on pregnant ruminants.
One feed plant that has the potential to serve as an anthelmintic is the jackfruit, as jackfruit leaves are used as an alternative forage during the dry season and they contain tannin antinutrient compounds. Tannin compounds in forages have a protective effect on the intestinal mucosa, so their consumption can reduce the population of parasites in the small intestine epithelial cells. The number of mast cells in the mucosa of the small intestine can be decreased by feeding tannins (Nguyen et al., 2013). Deworming programs often fail to control nematodes of the gastrointestinal tract because of the increasing prevalence of drug resistance (Mortemen et al., 2003). Kaplan (2004) reported that resistance to nematode populations is now recognized in many ruminants, such as sheep, goats, and cattle, as well as in monogastric animals, such as horses.
Actual cases now indicate that anthelmintics developed from tanniniferous plants can serve as potential strategic alternatives for controlling nematode infestations in small ruminants (Akkari et al., 2008). The leaves of jackfruit (Artocarpus heterophyllus) are now being studied as one of Indonesia’s potential medicinal plants. Jackfruit trees are widely grown, but the leaves are rarely used; therefore, jackfruit leaves have a high potential for use as an alternative anthelmintic. Plant tannins are reported to kill worms in livestock, so jackfruit leaves require further study as anthelmintic in farm animals.
The aim of the present study was to evaluate the in vitro potency of a jackfruit leaf extract as an anthelmintic agent in terms of its toxicity against adult H. contortus worms and its effects on worm morphometry. We hypothesized that a crude aqueous extract from A. heterophyllus leaves would show potential as an herbal anthelmintic against nematodes, especially H. contortus, under in vitro conditions.
MATERIALS AND METHODS
Tools and materials
The equipment for this study on changes in mortality in adult H. contortus worms included Petri dishes, a glass vessel for observing worms under a microscope, a microscope with a camera lucida to record the worms, and a stopwatch to measure the time of death of H. contortus after soaking in the jackfruit leaf crude aqueous extract. Other equipment included a Dutch oven and Erlenmeyer flasks for making the different doses of jackfruit leaf extract, an electric scale for determining the weight of jackfruit leaves, surgical scissors for removing worms from the abomasum of slaughtered goats, and micro calipers for calculating H. contortus morphometry.
The research materials included the crude extract of jackfruit leaves, adult H. contortus worms, distilled water, ethanol, and 0.62% NaCl solution.
Jackfruit Leaves Crude Extract
Jackfruit leaf extract was prepared at concentrations of 0.25%, 0.5%, 0.75%, 1%, and 1.25%, as described previously (Widiarso et al., 2018; Daryatmo et al., 2010). Briefly, the jackfruit extract was made by cutting the jackfruit leaves into small pieces, weighing out 1.25 g, 0.75 g, 0.5 g, and 0.25 g, and placing the pieces into separate beakers to prepare stock solutions. After adding 100 mL distilled water, the beakers were placed into an oven at 90 °C for 15 minutes. The liquid in the beakers was then filtered to obtain 0.25%, 0.5%, 0.75%, 1%, and 1.25% crude aqueous jackfruit leaf extracts.
Haemonchus contortus Collection
The H. contortus worms were obtained directly from the abomasums of naturally infected goats that had been slaughtered at a slaughterhouse. The abomasum was opened and the feces were carefully removed. Visible parasites were retrieved from the feces and stored in a container containing phosphate buffered saline (PBS) (Kuchai et al., 2012). The retrieved worms were then carefully broken up in a mortar to harvest the worm eggs. The worm eggs were suspended in PBS in a closed bottle and stored at room temperature for 3–5 days to maintain the worm eggs for treatment with jackfruit crude aqueous extract.
Worm Mortality Analysis
Treatments included 60 adult worms divided into 6 groups of 10 worms per treatment (Alemu et al., 2014). Each treatment consisted of five trials or five replications. Each group of worms was immersed in the jackfruit leaf crude extracts at different concentrations. Distilled water served as a negative control and albendazole as a positive control. Worm mortality was assessed every hour for up to 4 hours.
Data Analysis
Two-way ANOVA was used to analyze Haemonchus mortality for the various treatment doses and observation times, while one-way ANOVA was used to analyze the differences in morphometry of H. contortus.
RESULTS AND DISCUSSION
Anthelmintic Potential of Jackfruit Leaf Powder
Tannins, condensed tannins, saponins, and flavonoids are the active compounds contained in jackfruit leaf powder. The results of a quantitative analysis of jackfruit leaf powder are presented in Table 1.
Table 1: Analysis of compounds present in jackfruit leaf powder
| Sample | Result |
| Tannin Total | 23.56 mg/g |
| Condensed Tannin | 0.26 g/kg |
| Saponin | 0.97% b/b |
|
Flavonoid |
2.052.87 mg/kg |
Source: The Food and Agriculture Laboratory of the Faculty of Agricultural Technology, Gajah Mada University
Table 1 shows the results of laboratory tests confirming the high tannin content of jackfruit leaves. These leaves therefore have the potential to be anthelmintic, because Min and Hart (2003) demonstrated that a material with a tannin content of at least 5% has the capability of serving as an anthelmintic. According to Nora et al. (2017), tannin consumption by a ruminant can inhibit the development of nematode larvae, lower the adult worm population, reduce the ability of adult female worms to reproduce (frequency), and decrease the number of eggs released by the host animal through its feces. For example, the condensed tannin content of apus bamboo leaves was 0.26 g/kg. Condensed tannin levels are considered detectable at 0.2–2.5 g/kg, low if present at 2.5–10.0 g/kg, and medium if present at 10.0–50.0 g/kg (Jackson et al., 1996). Based on these ratings, the levels of condensed tannins in jackfruit leaves are low.
Haring et al. (2006) reported that condensed tannin concentrations of more than 55 g/kg of dry matter feed would reduce feed intake, decrease digestion rates, and lower the average daily weight gain of goats. A condensed tannin level of less than 50 mg/kg body weight in feed is effective against gastrointestinal parasites, as the tannins bind to the nematode wall protein, rendering the worms inactive so they die (Athanasiadou et al., 2001).
Haemonchus contortus mortality following administration of jackfruit crude leaf extract
The worm used in this study was Haemonchochus contortus, which is commonly found in the abomasum of small ruminants. The study examined 6 treatments with 3 repetitions; the results of the H. contortus mortality tests can be seen in Table 2. The tests indicated that the optimal test time was the second hour. The results for the test at the second hour can be seen in Tables 2 and 3.
Table 2: Mortality of Haemonchus contortus worms immersed in jackfruit leaf extract for 2 hours or 4 hours
| No | Treatment | 2 hours (%) | 4 hours (%) |
| 1 | Distilled water/negative control |
0±0.00a.h |
0±00a.h |
| 2 | 0.25% |
21.00±2.24b.h |
23.33±5.77b.h |
| 3 | 0.5% |
58.00±16.43c.h |
50±0.00c.h |
| 4 | 0.75% |
66.00±5.48d |
73.33±5.77d.i |
|
5 6 |
1% 1.25% |
72.00±8.37e 90.00±7.07f |
83.33±5.77e.i 100±0.00f.i |
| 7 | Albendazole/positive control |
86.00±11.40f |
100±0.00f.i |
a,b,c,d,e Different superscripts in one column indicate significant differences between treatments.
h,i Different superscripts in one line indicate a real difference between treatments.

Figure 1: Effects of jackfruit (Artocarpus Heterophyllus) leaf crude aqueous extract on the mortality of adult Haemonchus contortus worms.
Table 2 and Figure 1 shows that the results of the five repetitions of the Haemonchus contortus mortality test using jackfruit leaf extract were quite varied. A 2 hour treatment of deep soaking in crude aqueous Artocarpus heterophyllus extract at a dosage of 0.25% gave an average mortality rate of Haemonchus contortus worms of 21.00%, whereas a dosage of 0.5% gave an 58% mortality rate, a dosage of 0.75% gave a 66% mortality rate, a dosage of 1% gave a 72.00% mortality rate, and a dosage 1.25% gave a 90.00% mortality rate. The negative control (distilled water) gave an average mortality rate of 0.00%, while the positive albendazole control gave an average worm mortality rate of 86.00% (Figure 1).
Table 3: Effects of in vitro administration of jackfruit (Artocarpus heterophyllus) leaf crude aqueous extracts on the morphometry of adult female Haemonchus contortus worms
| Worm morphometry | Negative Control (0%) |
Heterophyllus leaf crude aqueous extract 0.25% |
Heterophyllus leaf crude aqueous extract 1.25% |
| Body length (mm) |
27.49±0.43a |
26.99±0.09b |
25.12±1.85c |
| Cervical papilla width(mm) |
0.39±0.01a |
0.34±0.01b |
0.31±0.02c |
| Vulval flap length (mm) |
4.67±0.06a |
4.10±0.04b |
3.83±0.06c |
a,b,cDifferent superscripts within the same row indicate significant differences (P<0.05)
rate of 90% after 2 hours and 100% after 4 hours, indicating that this dosage could replace chemical anthelmintics, such as albendazole, which had an 86.00% vermicidal action. Jackfruit leaf extract clearly showed anthelmintic ability against Haemonchus contortus. Nguyen et al. (2013) reported that forages, such as Gliricidia sepium, Caliandra calothyrsus (kaliandra), and Artocarpus heterophyllus (jackfruit leaves), in animal feed had anthelmintic activity against worms and coccidia. The best dose for mortality of adult H. contortus in the present study was 1.25%, because 100% of the worms were killed in 4 hours. The mortality relates to the tannin content in Artocarpus heterophyllus. Kamaraj et al. (2011) reported that tannins, saponins, flavonoids, and alkaloids are natural ingredients that have the ability to act as anthelmintics.
The Effect of Crude Aqueous Extracts of Jackfruit Leaves on the Morphometry of Adult Female Haemonchus contortus Worms
The Artocarpus heterophyllus leaf crude aqueous extract altered the morphometry of adult male and female Haemonchus contortus worms. Measurements included worm body length, cervical papilla width, and vulva flap length (Table 3 and Table 4).
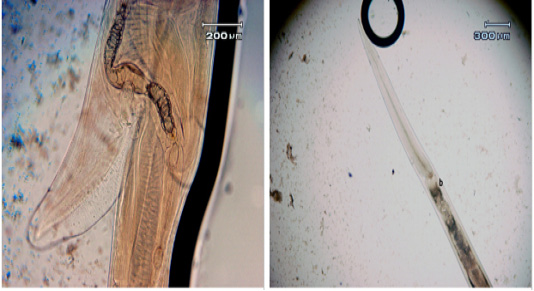
Figure 2: a. The length of the vulva flap; b. The width of the cervical papilla in adult female Haemonchus contortus worms
Table 4 shows a significant difference between the body length of adult female H. contortus worms treated at dosages of 0.25% and 1.25% compared to the control. The cervical papillae width showed no significant difference between the dosages of 1.25% versus the control, but a dose of 1% did show a significant difference. The length of the vulva did not differ significantly between dosages of 0.25% and the control, but a dosage of 1.25% resulted in a significant decrease compared to the control and the 0.25% dosage. Reductions in worm body length, the width of the cervical papilla, and the length of the vulva flap were caused by cuticle damage due to tannins contained in the jackfruit leaves. These tannins are able to bind proteins and inactivate the nematode wall, leading to worm death, as reported by Athanasiadou (2012). Molan et al. (2009) also showed that consumed tannins have a different effect on the growth of adult worms and larvae in ruminants. Hoste et al. (2006) revealed that tannins from jackfruit leaves can bind proteins and kill parasitic worms.
Table 4: Effects of in vitro administration of jackfruit (Artocarpus heterophyllus) leaf crude aqueous extracts on the morphometry of adult male Haemonchus contortus worms
|
Worm morphometry |
Control (0%) |
heterophyllus leaf crude aqueous extract 0.25% |
A. heterophyllus leaf crude aqueous extract 1.25% |
| Body length |
17.22± 0.22a |
16.28± 0.10b |
14.33± 0.13c |
| Cervical papilla width |
0.44± 0.01a
|
0.41± 0.01b
|
0.36± 0.04c
|
| Spicule length |
0.54± 0.01a |
0.44± 0.01b |
0.38± 0.01c |
a,b,cDifferent superscripts within the same row indicate significant differences (P<0.05).
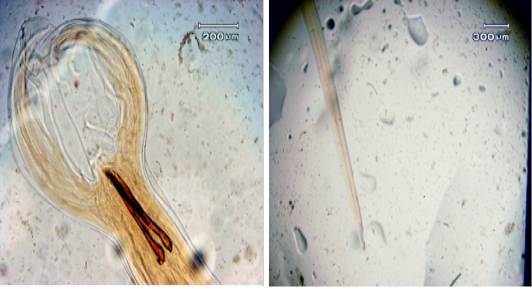
Figure 3: a. The length of the spicule; b. The width of the cervical papilla in adult male Haemonchus contortus worms
Microscopy observation showed differences in both male and female adult H. contortus worms. Dosages of 0.25% and 1.25% shortened the length of the body of adult male worms, with the length of adult male worms smaller than that of female worms (17.22 ± 0.22 vs. 27.49 ± 0.43 mm) (Figure 2). At both doses, significant decreases were observed for spicule length, cervical papillae width, and body length. The spicule length (Figure 3) was shorter in worms treated with the 1% dosage (0.38 ± 0.01) than with the 0.25% (0.44% ± 0.01) or with distilled water (0.54 ± 0.01), but the difference between the 0.25% dosage and water was not statistically significant. The width of the cervical papilla in adult male worms (Figure 3) was significantly smaller in the worms treated with the 1% dosage (0.36 ± 0.04) than with the 0.25% dosage (0.41% ± 0.01) and between the worms treated with the 1.25% dosage compared to the water controls (0.44 ± 0.01), but there was no significant effect between the control and the 0.25% dosage. The many differences in morphometry between the different doses compared to the control may reflect the influence of tannins, as reported for bamboo leaf infusions that can damage the cuticle of adult worms, disrupt the digestion process, and bind proteins to result in adult worm death (Kuchai et al., 2012). The jackfruit leaf extract used here caused many morphometric changes, possibly due to the influence of tannins, which can damage the cuticles of adult worms and interfere with the digestive process.
Sambodo et al. (2018) revealed that in vitro exposure to a Biophytum persianum crude extract rich in condensed tannins induced cuticle changes in H. contortus in the form of longitudinal and transverse wrinkles. H. contortus was described as having a cylindrical shape, a yellowish color, and an anterior end with a blunt point. The posterior end (bursa) of the adult male H. contortus appeared enlarged. The adult female was observed as having alternating red and white “pruning poles.” Small teeth were apparent in the buccal cavity, with those in males (17.3–20.0 mm; 0.24–0.33 mm) shorter and narrower than in females (25.5–32.6 mm; 0.38–0.63 mm) (Priyo et al., 2018). Martinez Ortiz-de-Montellano et al. (2013) found wrinkles on the cuticle and anterior end of H. contortus.
CONCLUSION
A jackfruit leaf aqueous crude extract caused significant differences in mortality rates, observation time, and morphometric measurements of adult H. contortus worms, especially in terms of body length, cervical papilla width, and vulva length in females and body length, cervical papilla width, and spicule length in males. The optimal dose of crude jackfruit leaf extract for H. contortus mortality was 1.25% in the present study. Based on the in vitro worm mortality and morphometry examinations, the Artocarpus heterophylus leaf aqueous crude extract appears to be a potential anthelmintic agent against Haemonchus contortus infestations.
ACKNOWLEDGMENT
The researchers would like to thank Indonesian Centre for Agriculture Education, Indonesian Agency for Agriculture Extension and Human Resourches Develpoment , Minisitry of Agriculture for providing research fund grant number 5892-01.1001052.EJ.522191.
CONFLICT OF INTEREST
The authors state that there is no conflict of interest.
AUTHORS CONTRIBUTION
BPW, DAD, KS and DEP the researchers. BPW is research conceptor,and managed all of research parts. DAD and KS prepared Haemonchus contortus, colected data for manuscript DEP managed and examined goat parasites in field and laboratory, assesed tanin, saponin, flavonoid and condensed tannin in Artocarpus heterophyllus.
REFERENCES
281-286.





