Advances in Animal and Veterinary Sciences
Research Article
Effect of Microencapsulated Allyl-Isothiocyanate on Survival of Salmonella Enteritidis and Enterotoxin Production in Ready to Eat Chicken Nuggets
Asmaa M. Sh. Fayed, Aalaa S.A. Saad*
Reference Lab for Examination of Food of Animal Origin, Food Hygiene Department, Animal Health Research Institute, ARC, Dokki, Giza, Egypt P.O. 12622, Giza.
Abstract | Foodborne diseases and poisoning are worldwide public health concerns. Salmonella food poisoning is one of the major causes of bacterial enteritis in many countries. Salmonella enterotoxin (Stn) belongs to the main virulence factors causing diarrhea. Allyl isothiocyanate (AITC) is known to possess strong antimicrobial activity, although, not widely used in food industry because of its strong odor, poor water solubility, and reactivity with natural food nucleophiles. A microencapsulation method was performed to overcome these obstacles. This study was designed to monitor the effects of microencapsulated AITC as a natural antibacterial agent on survival of S. Enteritidis and stn gene expression in ready to eat chicken nuggets at chilling and freezing preservation. The results indicated that microencapsulated AITC decreased S. Enteritidis viable count from 5.7 log cfu/g (at zero day) in nuggets to 3log cfu/g at chilling preservation and to 0.3 log cfu/g at freezing preservation. As well as, the effect of microencapsulated AITC on stn gene expression started at zero day (0.31) increased gradually till reach maximum effect at 3rd day (0.37) at chilling preservation. The effect of microencapsulated AITC at freezing preservation started at zero day along the preservation period reaching maximum effect at 12th week 0.94. It was found that AITC microencapsulated could be used in ready to eat chicken nuggets at chilling and freezing preservation to decrease or get rid of large numbers of S. Enteritidis and lower its stn toxin secretion without affecting on the sensory properties of the food”.
Keywords | Microencapsulated Allyl-Isothiocyanate, Salmonella enteritidis, stn Production, Chicken nuggets, Chilling and freezing preservation
Received | April 01, 2021; Accepted | May 21, 2021; Published | July 28, 2021
*Correspondence | Aalaa S. Saad, Reference Lab for Examination of Food of Animal Origin, Food Hygiene Department, Animal Health Research Institute, ARC, Dokki, Giza, Egypt, P.O. 12622, Giza; Email: alaa.samir87@yahoo.com
Citation | Fayed AMS, Saad ASA (2021). Effect of microencapsulated allyl-isothiocyanate on survival of salmonella enteritidis and enterotoxin production in ready to eat chicken nuggets. Adv. Anim. Vet. Sci. 9(9): 1442-1448.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1442.1448
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Shawkey et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Foodborne diseases and poisoning are the widespread and great public health concerns of the modern world. Both developed and developing countries are largely affected by foodborne infections. Foodborne diseases not only affect people’s health and well-being but also have an economic impact on individuals and the countries (Abd-Elghany et al., 2015).
Salmonella food poisoning is one of the major causes of bacterial enteritis in many countries. At least every year 1 in 10 people fall ill and 33 million of healthy life years are lost. Of the Salmonella serotypes, S. Typhimurium and S. Enteritidis are the most important agents of foodborne Salmonellosis in humans. It was estimated that approximately 75% of human salmonellosis cases were due to contaminated food products, such as beef, pork, poultry, and eggs (WHO, 2018).
chicken meat products are winning popularity because they represent quick easily prepared meat meals and solve the problem of the shortage in fresh meat of high price that is not within the reach of large numbers of families with limited income. Although the manufacturing process and the inherent characteristics of the final product provide barriers to microbial survival and growth (Calicioglu et al., 2002), extensive handling during chicken nuggets processing may increase the possibility of cross-contamination with pathogenic microorganisms such as Salmonella (USDA/FSIS, 2017).
Salmonella enterotoxin (Stn) belongs to the main virulence factors causing diarrhea. Previous studies have shown that Salmonella enterotoxin (Stn), has biological activity similar to that of the cholera toxin (CT) and Escherichia coli heat labile enterotoxin (LT-I) (Nakano et al., 2012).
Allyl isothiocyanate (AITC) is extracted from Armoracia rusticana and it could be extracted from other cruciferous plants by hydrodistillation (Wu et al., 2009), is known to possess strong antimicrobial activity, capable of killing fungal and bacterial pathogens on plant seeds, fresh produce, bread, meat, and cheese (Li et al., 2007; Lee et al., 2009). So, it might be a potential natural antimicrobial agent for food preservation.
AITC is thought to do its antimicrobial effect through bacterial cell membrane damage that lead to of essential cellular metabolites leakage (Lin et al., 2000). However, any application of AITC to food systems is limited due to its strong odor, poor water solubility, and reactivity with natural food nucleophiles (Chacon et al., 2006; Kim et al., 2008). To solve these problems, a microencapsulation method was considered. The microcapsules are wrapped with a polymer and bound together with a certain coating strength. Microencapsulation can provide protecting sensitive food components, masking taste and odor as well as promoting controlled lease of core materials (Ko et al., 2008).
Microencapsulation is a method in which tiny particles or droplets are surrounded by a coating wall, or are embedded in a homogeneous or heterogeneous matrix, to form small capsules. It can envelop a solid, liquid, or gaseous substance within another sub-stance in a very small sealed capsule. The core material is gradually diffused through the capsule walls, thereby offering controlled release properties under desired conditions. Therefore, microencapsulation technology can be used to deliver bioactive components, improving their handling properties (Bakry et al., 2016).
The aim of this study was to assess the antimicrobial potency of microencapsulated AITC against S. Enteritidis inoculated at two storage temperature in refrigerated, freezed ready to eat chicken nuggets and its effect on gene expression Patterns of Salmonella enterotoxin (Stn).
MATERIALS AND METHODS
Bacterial strains
Strain of Salmonella Enteritidis (MW362308) used in this experiment was get from the Media and Quality Assurance Unit, Reference Lab of Food Safety department, AHRI, ARC where was stored at -80oc. S. Enteritidis frozen culture was activated with inoculation in tryptic soya broth (TSB; Biomark laboratories) and incubated overnight at 37oC for 24 hours to reach a final concentration of approximately 108 CFU/mL (determined by plating serial dilutions on XLD agar, Merck): Serial dilution was performed on sterile PBS (phosphate buffer saline) to give approximately 105 CFU/mL in the injected solution.
Preparation and characterization of microparticles
Arabic gum aqueous solution (Food grade, Sigma), (25% w/w) was prepared by stirred overnight at room temperature. Then, AITC oil was mixed with the overnight aqueous solution with 0.5% tween 20, to reach ratio 3:1 of Arabic gum to AITC be. The emulsion was then homogenized using a high-speed mixer set at 11,000 rpm for 3 min. after that, the (2.5%, w/v) chitosan solution (chitosan dissolved in 50 mM lactic acid solution), was poured to this emulsion. Finally, the emulsion was made uniform at 11,000 rpm for 10 min, then the aqueous solution was sent to NAWAH company for microparticle lyophilization and Characterization (Scanning electron microscopy (SEM) (Gold coated scanning).
Release of AITC from microparticles
For evaluating the release of AITC from micro-particles, 0.1 g of powder was placed in 5 mL capped vial with diaphragm. The volume was about 1 mL with sodium phosphate citrate buffers (pH 4), and the vial capped with Teflon lined cap equipped septum. After that, the mixture was stored at 10 °C for 15 days and was sent to NAWA company for quantifying the amount of AITC maintained in the powder.
Antimicrobial activity of microencapsulated AITC
The antimicrobial activity patterns were determined by culturing S. Enteritidis planktonic (106 CFU/ ml)) in trypticase soy broth (TSB; Biomark laboratories) containing different concentrations of microencapsulated AITC at 37°C for 24 h. After cultivation different dilutions were plated on the trypticase soy agar (TSA; Oxoid) and incubated at 37°C for 24–48 h.
Preparation of Ready to eat chicken nuggets
Chicken breasts previously examined free from contamination with Salmonella species were sliced in a bowl chopper after trimming off the fat from them up into nugget-sized pieces for 2-3min. Ice flakes were added and was chopped again for 2 min. after that condiment and vegetable oil were added. The chopped chicken breasts divided to 2 parts one part was mixed with microencapsulated allyl isothiocyanate powder with continuous chopping till uniform batter mix formation and other part (blank) not mixed with anything (control). Chicken emulsion (~15 g) was packed compacted and coated with flour. Then, the Chicken emulsion pieces was immersed in the scrambled eggs. Finally, the chicken nuggets were dredged with into the breadcrumb mixture. They were fried in an oil heated to 190◦C and fry all sides 3 minutes each until golden brown colour was got. The nuggets were cooled to room temperature, freezed overnight at 20±1°C.
Inoculation procedures
Cells were grown to stationary phase on Tryptic Soy Broth (TSB; Biomark laboratories). A total of 0.2 ml of the S. Enteritidis strain was inoculated using a syringe in two spots (0.1 ml each) of the thawed chicken nuggets and grouped ; (group 1) nuggets with microencapsulated AITC inoculated with S. Enteritidis kept in refrigerator (chilling preservation), (group 2) nuggets without microencapsulated AITC inoculated with S. Enteritidis kept in refrigerator (group 3) nuggets with microencapsulated AITC inoculated with S. Enteritidis kept in freezer (freezing preservation) (group 4) nuggets without microencapsulated AITC inoculated with S. Enteritidis kept in freezer. (Group 4) nuggets with microencapsulated AITC not inoculated with S. Enteritidis for sensory evaluation of the product technology.
Salmonella enteritidis viable counts
Nugget groups were sampled after 2 h on day zero and then at 1st, 3rd 7th day of inoculation at chilled group and every week within the 1st month, every two weeks within the 2nd and 3rd months at freezing group. All tests were carried out in triplicate 15 g of each sample were homogenised using stomacher (Seward stomacher 80 Biomaster, England) with 135 mL of saline (CHEM LAB NV) obtaining 1:10 dilution. Tenfold serial dilutions of the homogenate prepared using in 0.1% sterile peptone water (LAB M) 1 mL sample suspension) was aseptically transferred to the dried surface of 3 XLD agar (Merck) plates at 0.4 mL, 0.3 mL, and 0.3 mL and the inoculum spread using sterile bent glass streaking rod. The plates were retained in upright position until the inoculum was absorbed by the agar and then inverted and incubated at 37oC for 24 hours to determine the total count of S. Enteritidis (by summation of S. Enteritidis in the three XLD agar (Merck) and multiplication by the sample dilution factor).
Gene expression assay
Extraction of mRNA was done using EasyPure®RNA kit, Transbionovo following its manufactur’s instructions. The extracted RNA was added to a reverse transcriptase RT buffer master mixture of and primer mix RT containing SYBR® Green I dye and incubated at 42 °C for 15 min, followed by further incubation at 85 °C for 5 s to inactivate the reverse transcriptase.
The synthesized oligonucleotide primers (Integrated DNA Technologies Inc., Coralville, IA, USA) were used in this study (Table 1).
The relative expression of targeted genes was estimated by the comparative cycle threshold method (Livak and Schmittgen, 2001). The cycle threshold values of the target genes belonged to S. Entritidis obtained from the micro-encapsulated AITC containing samples and blank sample (control) were compared and normalized to the endogenous reference gene (16S rRNA)
Sensory evaluation of ready to eat chicken nuggets containing microencapsulated AITC
The evaluators were 12 specialists from the staff members of Food Hygiene Department, Animal Health Research Institute, ARC. Ready to eat chicken nuggets containing microencapsulated AITC, and blank (not contain microencapsulated AITC) were tested and the most frequently cited descriptors (color, taste, flavor and overall acceptance). The specialists were approached to rank the samples according to their intensity for every one of the four descriptors.
There was 9-point hedonic scale where 9 means (Kim et al., 2015); “Like Extremely” of the nuggets and one means; “Dislike Extremely” of the nuggets as compared to nuggets free from microencapsulated AITC. When the stated scores were under point five, the auditor considered sample rejected.
Table 1: Primer sequences used in RT-PCR analysis for S. Enteritidis.
| Gene | Molecular function | Primer name and sequence | References |
|
16S sal. |
Endogenous Reference Gene | F: AGGCCTTCGGGTTGTAAAGT |
Xu et al. (2010) |
| R: GTTAGCCGGTGCTTCTTCTG | |||
|
Stn sal. |
Salmonella enterotoxin | F: GCCATGCTGTTCGATGAT | Moore and Feist (2007) |
| R: GTTACCGATAGCGGGAAAGG |
Statistical analysis
All analyses were performed in three replicates. Data were analyzed using the Statistical excel (Microsoft, 2013). Significant differences among the results were examined by the T-student test at α=0.05. The data were expressed as the means ± SD.
RESULTS
Preparation and characterization of microencapsulated AITC
Microencapsulated AITC had a rounded external surface with concavities and dents Figure 1. The particle size of the microparticles ranged from 0.9 um to 5.1 um in a percentage of 88.7% and 11.3% respectively Figure 1. AITC release from the gum Arabic–chitosan microparticles. Approximately 20% and 15% of the loaded AITC was released from the microparticles during storage for 7days of chilling preservation and 90 days of freezing preservation respectively.
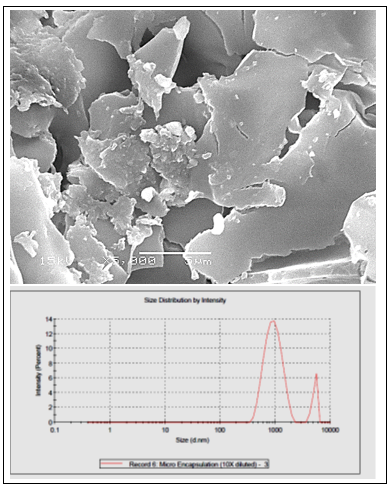
Figure 1: Scanning electron micrograph showing the appearance of microencapsulated AIT. Samples were examined on a cold stage at 5000X (A). Size Distribution by Intensity (B).
Antimicrobial activity of microencapsulated AITC
The antimicrobial effect of microencapsulated AIT against S. Enteritidis was apparent at the highest concentration used 5000ppm.
Effect of microencapsulated AITC on S. Enteritidis viable count inoculated in ready to eat chicken nuggets
The effect of microencapsulated AITC on S. Enteritidis viable count inoculated in ready to eat chicken nuggets at chilling preservation expressed as log cfu/g showed on Figure 2. The starting count (at Zero day), of control and treated ready to eat chicken nuggets with microencapsulated AITC are 5.7 log cfu/g each. S. Enteritidis viable count in treated nuggets with microencapsulated AITC decreased gradually at chilling than control significantly (P < 0.05) to 3log cfu/g at the 7th day of preservation.
Figure 3 shows S. Enteritidis viable count inoculated in ready to eat chicken nuggets at freezing preservation. At zero day, the initial viable count of control and treated microencapsulated AITC ready to eat chicken nuggets were 5.4 log cfu/g and 5.3 log cfu/g, respectively, while through the whole freezing preservation period the viable count of treated samples decreased than starting count by 5logs significantly (P < 0.05) to 0.3log cfu/g. Also, S. Enteritidis viable count in treated nuggets with microencapsulated AITC decreased than the control group at all examined sample at the same time at both chilling and freezing preservation.
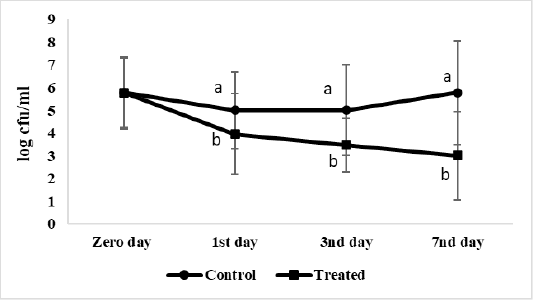
Figure 2: Salmonella Enteritidis viable count (log cfu/g) of ready to eat chicken nuggets treated with of microencapsulated AITC and control (untreated) at chilling preservation. Values are expressed as the mean ± SD. There are significant differences between points that have different letters on the same day of preservation.
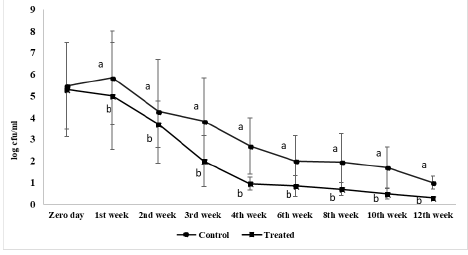
Figure 3: Salmonella Enteritidis viable count (log cfu/g) of ready to eat chicken nuggets treated with of microencapsulated AITC and control (untreated) at freezing preservation. Values are expressed as the mean ± SD. There are significant differences between points that have different letters on the same day of preservation.
Results of gene expression assay
Results of relative expression of S. Enteritidis stn gene in ready to eat chicken nuggets treated with microencapsulated AITC at chilling and freezing preservation are shown in Figures 4 and 5, It was found the relative expression of the stn gene was decreased compared to control at both preservation types (chilling and freezing). It was noticed at that the effect of microencapsulated AITC on stn gene expression started at zero day (0.31) increased gradually till reach maximum effect at 3rd day (0.37) of preservation then gradual decrease at 7th day (0.29) of chilling preservation.
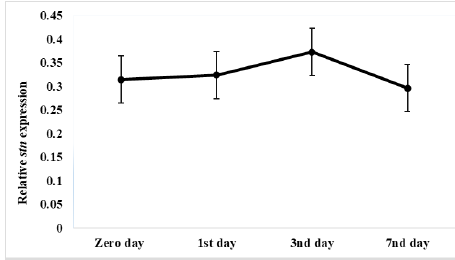
Figure 4: Relative stn gene expression of Salmonella Enteritidis in ready to eat chicken nuggets treated with of microencapsulated AITC at chilling preservation.
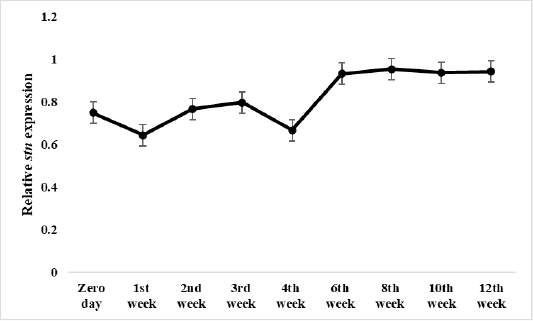
Figure 5: Relative stn gene expression of Salmonella Enteritidis in ready to eat chicken nuggets treated with of microencapsulated AITC at freezing preservation.
The effect of microencapsulated AITC at freezing preservation started at zero day along the preservation period reaching maximum effect at 12th week 0.94 the end of preservation.
Result of sensory evaluation of ready to eat chicken nuggets containing microencapsulated AITC
The sensory evaluation results of ready to eat chicken nuggets containing microencapsulated AITC are shown in Figure 6. It shows the results for five sensory evaluation items (color, taste, flavor, and overall acceptability) which gave high score that was 8.1, 8, 8.4, 8.3 respectively.
Discussion
The shapes and morphology of the microencapsulated AITC (Gum Arabic–chitosan microparticles) was near to mentioned previously of (Chacon et al., 2006; Ko et al., 2012), its shape may be gained due to diminishment of the gum Arabic particles during drying.
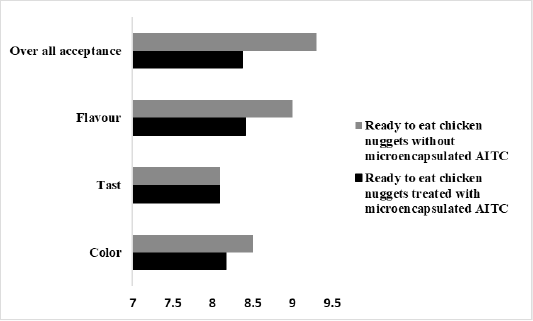
Figure 6: The average score of taste, color, flavor, and the overall acceptability of in ready to eat chicken nuggets without microencapsulated AITC and ready to eat chicken nuggets treated with of microencapsulated AITC.
Allyl isothiocyanate (AITC), was used to ensure quality and safety of food, it was used for its antimicrobial effect against some pathogenic bacteria yeasts, or molds. Although its use of food systems is restricted due to its strong odor and its volatile nature (Jin and Gurtler, 2011).
The present study showed that high level of AITC was needed to achieve the bactericidal effects against S. Enteritidis this result matched with Chacon et al. (2006) who reported that higher level of AITC was effective against E. coli O 157. At both preservation (chilling and freezing) the microencapsulated AITC had great effect on decrease S. Enteritidis count but not had bactericidal effect this may be due to That interaction of AITC with meat constituents may decrease AITC antimicrobial activity. Also, AIT is decomposed through nucleophilic attack by water and hydroxide ions in aqueous media Pechacek et al. (1997) As well as, AIT faster depletion due to its reaction with thiols, sulphydryls, and free amino groups of proteins, in meat systems (Ward et al., 1998; Nadarajah et al., 2005). The inhibition of virulence gene expression by plant extracts and plant essential oils has been verified for many pathogens, such as Staphylococcus aureus, E. coli and Candida albicans (Qiu et al., 2011a; b; Raut et al., 2017). Thus, the expression of the stn gene decreased in both preservation types (chilling and freezing) in treated microencapsulated AITC group with in comparison to the control group. This result similar to (Zou et al., 2012) who mentioned that the relative expression level of stn gene in salmonella treated AITC was down-regulated in both planktonic and biofilm cells.
Addition of microencapsulated AITC to ready to eat chicken nuggets appeared not affect average score of taste, color, flavor, and the overall acceptability of the product against chicken nuggets without microencapsulated AITC (Figure 6) that the microencapsulation overcome strong odor and volatile nature of AITC this result was conducted to (Ko et al., 2012) who mentioned that microencapsulated AITC not affect sensory evaluation of Kimchi. This make it is able to make use of AITC in food systems.
CONCLUSION AND RECOMMENDATIONS
This study was performed to observe the effects of microencapsulated AITC as a natural antibacterial S. Enteritidis and stn gene expression in ready to eat chicken nuggets at chilling and freezing preservation. microencapsulated AITC addition to ready to eat chicken nuggets resulted in positive changes in inhibition of S. Enteritidis and stn gene expression at 2 preservation conditions. S. Enteritidis inoculated at 5.7 log cfu/g nuggets with microencapsulated AITC by decreased to 3log cfu/g at chilling preservation and 0.3log cfu/g at freezing preservation. The effect of microencapsulated AITC on stn gene expression started at zero day (0.31) increased gradually till reach maximum effect at 3rd day (0.37). The effect of microencapsulated AITC at freezing preservation started at zero day along the preservation period reaching maximum effect at 2nd week 0.35. These results constitute a theoretical foundation for the application of AITC as a nature additive in the food industry.
ACKNOWLEDGMENTS
We would like to thank Reference Lab for Examination of Food of Animal Origin, food hygiene department and Poultry Diseases Departments, Animal Health Research Institute, ARC, Giza, Egypt for support.
NOVELTY STATEMENT
Allyl isothiocyanate (AITC), was known about its antimicrobial effect against some pathogenic bacteria yeasts, or molds. Although its use of food systems is restricted due to its strong odor and its volatile nature. various studies have shown that the it could be used in food system after some modification. The present study ensures its antibacterial effect on one of pathogenic bacteria Salmonella and also its ability on stn gene expression in ready to eat chicken nuggets at chilling and freezing preservation.
AUTHOR’S CONTRIBUTION
All authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES





