Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (2): 120 – 123A Rapid Multiplex PCR Method for the Diagnose of Freemartin Syndrome in Domestic Cattle (Bos taurus)
Miguel Angel Ayala–Valdovinos1*, Jorge Galindo–García1, David Sánchez–Chiprés1, Theodor Duifhuis–Rivera1, Clemente Lemus–Flores2,
- Instituto de Biotecnología Animal, Departamento de Producción Animal, División de Ciencias Veterinarias, Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara, Predio las Agujas, C.P. 45200, Zapopan, Jalisco, México
- Laboratorio de Genética Molecular, Universidad Autónoma de Nayarit, Posgrado CBAP, Ciudad de la Cultura Amado Nervo, C.P. 63155 Tepic, Nayarit, México
*Corresponding author: manayala@cucba.udg.mx
ARTICLE CITATION:
Ayala–Valdovinos MA, Galindo–García J, Sánchez–Chiprés D, Duifhuis–Rivera T, Lemus–Flores C (2014). A rapid multiplex PCR method for the diagnose of freemartin syndrome in domestic cattle (Bos taurus). Adv. Anim. Vet. Sci. 2 (2): 120 – 123.
Received: 2013–12–17, Revised: 2014–01–09, Accepted: 2014–01–09
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.2.120.123
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
The majority of heifer calves born as hetero–sexual twin births are sterile freemartins. It is important that this condition be diagnosed at an early age because freemartin heifers can’t be used as replacement stock. Different methods are available for the diagnosis of freemartinism, however molecular methods are preferred due to their accuracy and shorter duration of process. In the present study, freemartinism status of 40 female calves of heterosexual multiple births was investigated by multiplex polymerase chain reaction (multiplex PCR). The reaction was carried out using two primer sets for the Sry and K–casein genes of domestic cattle. The PCR product was analyzed by agarose gel (3%) electrophoresis, which allowed to identify the genotypes of the animals studied: normal males (453 bp and 163 bp), normal females (453 bp), and 39 (97.50%) study cases that showed chromosome chimerism (freemartin, 453 bp and 163 bp). Compared to the polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) technique, this method (i.e., multiplex PCR) proved a far cheaper and quicker (approximately 27 minutes) way to diagnose freemartinism.
INTRODUCTION
The term freemartin is used to refer a sterile heifer (farrow = infertile animal and mart = heifer), and is by extension, used to refer to a genetic female from a heterosexual multiple birth. In over 90% of such cases, chorionic vascular anastomoses connecting the placentas of the twin fetuses occur at 30 to 40 days of gestation, i.e., before sexual dimorphism takes place. This results in an interchange of cells (hematopoietic chimerism) and plasma substances such as hormones between the twin embryos, leading to an intersexual state in the female (Harvey, 1976; Gustavsson and Johansson, 1980; Long, 1990). This intersexual condition is characterized by external genitalia that are essentially female in appearance, and internal genitalia that are affected to varying degrees, typically involving gonadal hypoplasia, inhibition of the Müllerian ducts, masculinization of the gonads, and stimulation of the Wolffian ducts (Tran et al., 1977; Edwards et al., 1994; Harikae et al., 2012).
The incidence of freemartin syndrome in heterosexual bovine twins has been estimated to be 92% (McFeely et al.,, 1967; Marcum 1974; David et al., 1976). However, freemartins can also occur in single female births, as a result of the early fetal death and reabsorption of the male twin within the uterus after the development of vascular anastomoses and once sexual differentiation has occurred (Smith et al., 1977). The basic techniques traditionally used to diagnose freemartin syndrome have included clinical examination, tolerance to homo–grafts test, blood typing, and cytogenetic analysis. However, the advent of the polymerase chain reaction (PCR) technique and the use of specific DNA sequences of the bovine Y chromosome constitute significant advances in the detection of freemartins, particularly when few of the calf’s cells have an XY chromosome complement. Compared to the polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) analysis, the multiplex polymerase chain reaction (multiplex PCR) method proved a far cheaper (not using restriction enzyme) and quicker (no time restriction) way to diagnose freemartinism (Olsaker et al.,. 1993; Ennis et al.,. 1999; Padula, 2005).
MATERIALS AND METHODS
Animals and Samples
A total of 40 Bos taurus calves (31 Holstein, 4 American Brown Swiss, 2 Jersey, and 3 Simmental), all products of multiple heterosexual births, were selected to be tested for freemartin syndrome. The clinical history of each animal
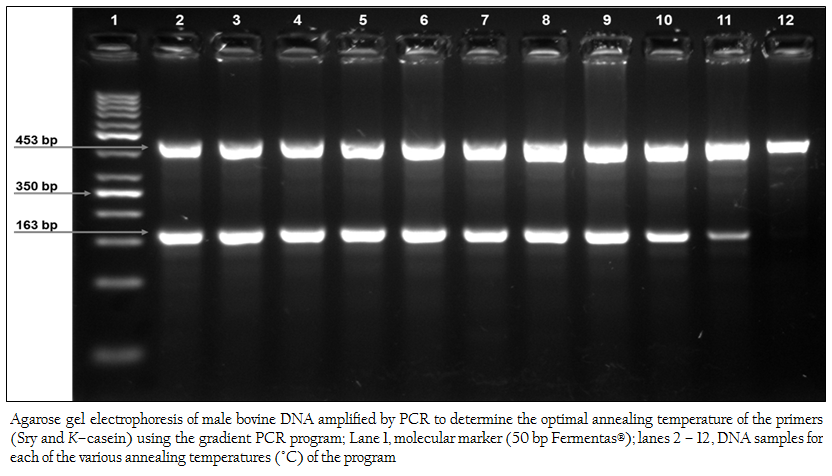
Figure 1:Agarose gel electrophoresis of male bovine DNA amplified by PCR to determine the optimal annealing temperature of the primers (Sry and K–casein) using the gradient PCR program; Lane 1, molecular marker (50 bp Fermentas®); lanes 2 – 12, DNA samples for each of the various annealing temperatures (°C) of the program
was taken and blood sample was collected for molecular analysis by jugular venipuncture using a vacutainer needle and vacuum tubes containing 1.5 mg/mL of EDTA.
DNA Extraction
DNA was isolated from the blood samples of each animal; 100 L of blood was mixed with 900 L of buffer A (0.32 M sucrose; 10 mM Tris HCI; 5 mM MgCl2; 1% Triton X–100). The mixture was then centrifuged at 12,000 rpm for 2 minutes and the supernatant then removed; this process was repeated until a white cell pellet was formed. Next, the sample was incubated for one hour at 50ºC in a Proteinase K solution (8 mg/mL) in buffer D (50 mM KCl; 10 mM Tris–HCl; 2.5 mM MgCl2; 0.455 NP–4O, 0.45% Tween–20). The Proteinase K was inactivated by incubating the sample at 90ºC for 10 minutes (Ayala–Valdovinos et al., 2007).
Gradient PCR
Gradient PCR, using the nucleotide sequence of the Sry gene (GenBank accession number NM_001046429.2) and K–casein gene (GenBank accession number BC102120.1) of bovine, was performed. For each gene were employed two previously reported oligo–nucleotide primers (5´–TGAACGCTTTCATTGTGTGGTC–3´ and R 5´–GCCAGTAGTCTCTGTGCCTCCT–3´ for the Sry–F gene (Pomp et al., 1995); and F 5´– TGTGCTGAGTAGGTATCCTAGTTATGG–3´ and R 5´–GCGTTGTCTTCTTTGATGTCTCCTTAG–3´ for the K–casein gene (Barroso et al., 1997); followed by a standard PCR protocol (final volume 20 L), with 100 ng of DNA, 0.50 U of Taq DNA Polymerase, 0.2 mM of dNTPs, 0.4 M of each primer, 1X of PCR buffer, 1.5 mM of MgCl2, and 10.55 L of ddH2O. The amplification cycles were performed in a gradient thermal cycler (TC–5000, Techne, USA) with 40 cycles of denaturation at 94°C for 60 seconds, the annealing temperature having been previously determined by the equipment’s gradient program; the extension temperature was 72°C for 60 seconds.
Subsequently, the amplification product was analyzed by GelRed–stained agarose gel electrophoresis with a 1X TBE buffer solution (Rickwood and Hames, 1990). Lastly, the DNA bands were observed and photographed under UV light in a gel documentation system (Kodak Gel Logic 200).
Multiplex PCR
Based on the gradient PCR, was selected the annealing temperature (54°C) for performing multiplex PCR with the chosen Sry and K–casein gene primers. For a fast multiplex PCR protocol, a high–speed thermal cycler (Thermo Scientific Piko, Finland) and 0.2 mL ultra–thin–walled PCR tubes (Thermo Scientific) were used as follows: 95°C for 2 minutes, followed by 32 amplification cycles at 94°C for 5 seconds, 54°C for 5 seconds, 72°C for 15 seconds, and a final extension of 72°C for 3 minutes.
RRESULTS
The optimal annealing temperature of the oligo–nucleotide primer used in this study (Sry and K–casein) was determined by using the gradient PCR program (Techne gradient thermal cycler, UK). Figure 1 shows the electrophoretic run of 11 samples containing DNA from the same male bovine control specimen, which were amplified at a different annealing temperature in accordance with the equipment’s program (lane 2, 50.2°C; lane 3, 50.8°C; lane 4, 52.3°C; lane 5, 54°C; lane 6, 55.5°C; lane 7, 57.2°C; lane 8, 58.8°C; lane 9, 60.7°C; lane 10, 62.2°C; lane 11, 63.9°C; lane 12, 65°C). A wide range of optimal annealing temperatures for the primers were found (lanes 2 – 9). Based on these experiments, the annealing temperature selected for this pair of primers was 54°C (lane 5). With the primers used in this study, through the dilution of male bovine blood in the female bovine blood, it was shown that XY cells were detectable up to a concentration of 0.1%.
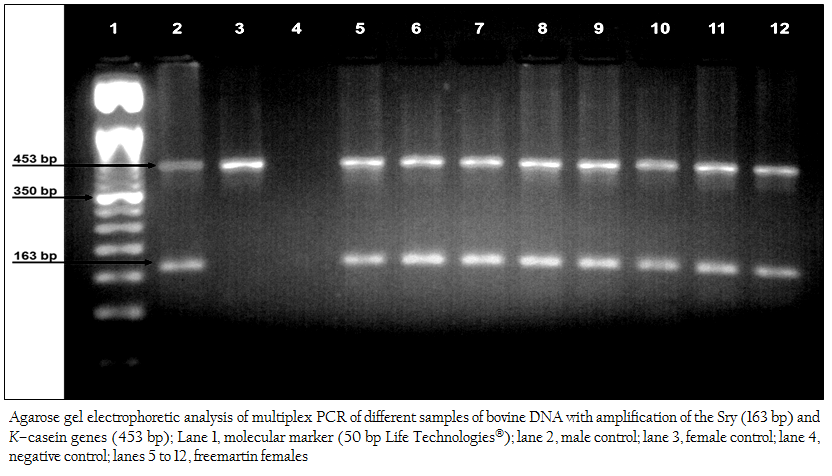
Figure 2: Agarose gel electrophoretic analysis of multiplex PCR of different samples of bovine DNA with amplification of the Sry (163 bp) and K–casein genes (453 bp); Lane 1, molecular marker (50 bp Life Technologies); lane 2, male control; lane 3, female control; lane 4, negative control; lanes 5 to 12, freemartin females.
The fast multiplex PCR method taking approximately 27 minutes allowed the amplification of the genomic DNA fragments corresponding to the Sry (163 bp) and K–casein (453 bp) genes of the cattle The product of the multiplex PCR was analyzed by agarose gel (3%) electrophoresis stained with ethidium bromide, allowing to identify the genotypes of the animals tested: normal males (453 bp and 163 bp), normal females (453 bp), and freemartins (453 bp and 163 bp) (Figure 2), which resulted in the diagnosis of 39 (97.50%) cases of intersex conditions due to freemartin syndrome, in a sample of 40 heifers born of heterosexual multiple births.
DISCUSSION
Freemartins display certain clinical signs, particularly in terms of their genitalia, though the appearance of the external genitalia of the newborn calf is sometimes relatively normal. In certain cases, a freemartin calf displays elongation of the clitoris and a tassel of hair on the lower commissure of the vulva; however, these features are inconsistent and not particularly reliable for diagnostic purposes (Long, 1990; Padula, 2005; Sohn, 2007).
The homograft tolerance test is a complicated procedure and the male twin is not always available, therefore laboratory testing is necessary. The cytogenetic analysis for diagnosing freemartinism identifies the possible chimeric condition (XX/XY) in either heterosexual twin. Ideally, however, both twins should be sampled given their tendency to exhibit similar proportions of cells. In cases where XX/XY chimerism has occurred with relatively low frequency of XY cells, it is important to examine many metaphase spreads to be certain of the diagnosis of freemartinism. Statistically, 90 XX cells must be counted before we can be 99% certain that no XY cells are present in the female (Harvey, 1976; Long, 1990; Padula, 2005). Furthermore, poor–quality blood samples for the culture of lymphocytes prevent the collection of suitable metaphases for the cytogenetic analysis of freemartin syndrome (McNiel et al., 2006).
Male–specific DNA amplification methods, based on polymerase chain reaction (PCR), have particularly been used for sex chromosomal chimerism analysis in peripheral leukocytes because of their high sensitivity and rapidity (Hirayama et al., 2007). The present study demonstrates that the use of multiplex PCR is more efficient, less expensive, and more practical for routine early diagnosis of the bovine freemartins. This study allowed us to identify 39 (97.50%) freemartin heifers using a fast multiplex PCR technique involving the use of oligonucleotide primers for the Sry and K–casein genes, which clearly underlines the high frequency of the syndrome reported in the literature in cases of multiple heterosexual births in this species.
CONCLUSION
Molecular diagnostics (PCR–RFLP or Multiplex PCR) provides a useful tool with which to reach an accurate and timely diagnosis of the most commonly found intersex condition in domestic cattle, that is, freemartin syndrome, thereby making it possible to identify animals that would have no reproductive or productive value. Compared to the PCR–RFLP technique, this multiplex PCR technique offers a far cheaper and quicker (i.e., around 27 minutes) means of diagnosing freemartinism.
ACKNOWLEDGEMENTS
This study was fully sponsored by Universidad de Guadalajara with grant number P3E 137325.
REFERENCES
Ayala–Valdovinos MA, Villagomez DAF, Galindo–Garcia J, Sanchez–Chipres D, Avila–Figueroa D (2007). Anatomopathologic, cytogenetic and molecular studies of the freemartin syndrome in cattle (Bos taurus). Redvet. 9: 1 – 15.
Barroso A, Dunner S, Canon J (1997). Use of a single–strand conformation polymorphism analysis to perform a simple genotyping of bovine kappa–casein A and B variants. J. Dairy Res. 64: 535 – 540.
http://dx.doi.org/10.1017/S0022029997002471
PMid:9403768
David JS, Long SE, Eddy R (1976). The incidence of freemartins in heifer calves purchased from markets. Vet. Record. 21: 417 – 418.
http://dx.doi.org/10.1136/vr.98.21.417
Edwards JF, Gallagher DS, Prakash B (1994). Urethral atresia with uroperitoneum in a newborn bovine freemartin. Vet. Pathol. 31: 117 – 119.
http://dx.doi.org/10.1177/030098589403100118
PMid:8140717
Ennis S, Vaughan L, Gallagher TF (1999). The diagnosis of freemartinism in cattle using sex–specific DNA sequences. Res. Vet. Sci. 67: 111 – 112.
http://dx.doi.org/10.1053/rvsc.1998.0286
PMid:10425251
Gustavsson I, Johansson I (1980). Chromosome aberrations and their influence on the reproductive performance of the domestic animals. Z. Tierzuchtg. Zuchtgsbiol. 97: 176 – 195.
http://dx.doi.org/10.1111/j.1439-0388.1980.tb00925.x
Harikae K, Tsunekawa N, Hiramatsu R, Toda S, Kurohmaru M, Kanai Y (2012). Evidence for almost complete sex–reversal in bovine freemartin gonads: formation of seminiferous tubule–like structures and transdifferentiation into typical testicular cell types. J. Rep. Dev. 58: 654 – 660.
http://dx.doi.org/10.1262/jrd.2012-070
PMid:22813600
Harvey MJ (1976). Veterinary cytogenetics. Vet. Rec. 98: 479 – 481.
http://dx.doi.org/10.1136/vr.98.24.479
PMid:969170
Hirayama H, Katagiri S, Kageyama S, Minamihashi A, Moriyasu S, Sawai K, Onoe S, Takahashi Y (2007). Rapid sex chromosomal chimerism analysis in heterosexual twin female calves by Loop–mediated Isothermal Amplification. Anim Reprod Sci. 101: 38 – 44.
http://dx.doi.org/10.1016/j.anireprosci.2006.09.006
PMid:17011732
Long SE (1990). Development and diagnosis of freemartinism in cattle. In Pract. 12: 208 – 210.
http://dx.doi.org/10.1002/dc.2840060104
Marcum JB (1974). The freemartin syndrome. Anim. Breed Abstr. 42: 227 – 242.
McFeely RA, Hare WCD, Biggers JD (1967). Chromosome studies in 14 cases of intersex in domestic mammals. Cytogenetics. 6: 242 – 253.
http://dx.doi.org/10.1159/000129945
PMid:6068573
McNiel EA, Madrill NJ, Treeful AE, Buoen LC, Weber AF (2006). Comparison of cytogenetics and polymerase chain reaction based detection of the amelogenin gene polymorphism for the diagnosis of freemartinism in cattle. J. Vet. Diagn. Invest. 18: 469 – 472.
http://dx.doi.org/10.1177/104063870601800508
PMid:17037616
Olsaker I, Jorgensen CB, Hellemann AL, Thomsen PD, Lie O (1993). A fast and highly sensitive method for detecting freemartinism in bovine twins using immunomagnetic beads and Y–specific PCR primers. Anim. Genet. 24: 311 – 313.
http://dx.doi.org/10.1111/j.1365-2052.1993.tb00319.x
PMid:8239077
Padula AM (2005). The freemartin syndrome: An update. Anim. Rep. Sci., 87: 93 – 109.
http://dx.doi.org/10.1016/j.anireprosci.2004.09.008
PMid:15885443
Pomp D, Good BA, Geisert RD, Corbin CJ, Conley AJ (1995). Sex identification in mammals with polymerase chain reaction and its use to examine sex effects on diameter of day–10 or –11 pig embryos. J. Anim. Sci. 73: 1408 – 1415.
PMid:7665371
Rickwood D, Hames BD (1990). Gel Electrophoresis of Nucleic Acids: A Practical Approach, 2nd ed. IRL Press, Oxford, USA: Oxford University Press.
Smith GS, Van Camp SD, Basrur PK (1977). A fertile female co–twin to a male calf. Can. Vet. J. 18: 287 – 289.
PMid:922647 PMCid:PMC1697683
Sohn SH, Cho EJ, Son WJ, Lee CY (2007). Diagnosis of bovine freemartinism by fluorescence in situ hybridization on interphase nuclei using a bovine Y chromosome–specific DNA probe. Theriogenology. 68: 1003 –1011.
http://dx.doi.org/10.1016/j.theriogenology.2007.06.022
PMid:17870153
Tran D, Muesy–Dessole N, Josso N (1977). Anti–Mullerian hormone is a functional marker of foetal Sertoli cells. Nature. 269: 411 – 412.
http://dx.doi.org/10.1038/269411a0
PMid:909589




