Advances in Animal and Veterinary Sciences
Research Article
Detection of Anaplasmosis and Ehrlichiosis in Blood of Owned Dogs in Alexandria, Northern Egypt
Khaled Mohamed El-Dakhly1*, Magdy M. Tawfik1, Amany Samir Aboshinaf2, Lilian N. Mahrous1, Waleed M. Arafa1
1Department of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Provincial Laboratory of Animal Health Research Institute, Agriculture Research Center, Dokki, Giza, Egypt.
Abstract | Anaplasmosis and ehrlichiosis are important tick-borne diseases in dogs. The current study aimed to evaluate the occurrence of anaplasmosis and ehrlichiosis in owned dogs in Alexandria, northern Egypt. Accordingly, a total of 70 dogs, admitted to private clinics for veterinary care, were surveyed and blood specimens were taken for both Giemsa-stained microscopic examination and molecular detection using conventional PCR. The present study revealed that 8.57% (6/70) dogs were infected with Anaplasma/Ehrlichia spp. Based on the examination of Giemsa-stained blood smears, Anaplasma platys was found in blood platelets of 4 (5.71%) dogs and Ehrlichia canis was revealed inside macrophages of 2 (2.86%) dogs. No mixed infections were recovered. All infected dogs had an infestation with Rhipicephalus sanguineus ticks. Variable clinical signs were detected in the surveyed dogs, often loss of appetite, loss of body weight and lethargy. Among risk factors, it has been found that dogs aged less than 2 years were the highest infected as well as male dogs were more susceptible to the infection. cPCR-based molecular investigation targeting the 16sRNA gene revealed that 20.0% (14/70) of dogs were positive for Anaplasma/Ehrlichia species with a specific amplicon size of 345 bp. Expectedly, the use of PCR-based molecular tool was more accurate than the conventional microscopic examination. Further literature based on both traditional and molecular tools targeting another hemoparasites infecting dogs together with an accurate identification of the associated tick vector are urgently needed to achieve a successful control of those pathogens.
Keywords | Anaplasma platys, Ehrlichia canis, Dogs, Microscopy, PCR, Egypt
Received | March 05, 2021; Accepted | May 25, 2021; Published | July 28, 2021
*Correspondence | Khaled Mohamed El-Dakhly, Department of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; Email: eldakley_s71@yahoo.com
Citation | El-Dakhly KM, Tawfik MM, Aboshinaf AS, Mahrous LN, Arafa WM (2021). Detection of anaplasmosis and ehrlichiosis in blood of owned dogs in Alexandria, Northern Egypt. Adv. Anim. Vet. Sci. 9(9): 1383-1389.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1383.1389
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 El-Dakhly et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Family Anaplasmataceae (Order Rickettsiales) are obligate intracellular pathogens capable of infecting mostly mononuclear phagocytes of dogs and cats. Members of such family replicate within a vacuole derived from the host cell membrane (Dumler et al., 2001; Ybanez et al., 2012). In the beginning of 21st century, based on phylogenetic analyses, those species were reclassified, and the family included the genera Anaplasma, Ehrlichia, and Neorickettsia with various members were renamed, like Ehrlichia platys which is renamed to Anaplasma platys (Allison and Little, 2013).
Canine vector-borne diseases constitute a major veterinary health obstacle in Mediterranean districts (Hamel et al., 2012). Among the causative agent of those, both Anaplasma platys and Ehrlichia canis are potential pathogens of veterinary concern (Ebani, 2019). Anaplasma platys was first detected in 1978 in Florida, USA, and it was initially known as Ehrlichia platys. It replicates inside blood platelets causing a symptomatic condition in infected dogs, however, life-threatening pancytopenia, (canine infectious cyclic thrombocytopenia, CICT), via damage of platelets due to severe anaplasmosis, has been reported (Huang et al., 2005; Aguirre et al., 2006; Ramos et al., 2014).
Ehrlichia canis was first detected in 1935 in Algeria and it has a great affinity to monocytes and macrophages with acute clinical symptoms mostly fever and anemia in infected dogs (Aguirre et al., 2004) causing tropical canine pancytopenia, recently, canine monocytic ehrlichiosis (CME) in both tropical and subtropical countries (Harrus and Waner, 2011; Ramos et al., 2014; Sainz et al., 2015; Malik et al., 2018). E. canis often infect dogs, although literature denoted the infection in cats (Ebani et al., 2017). Moreover, human infections associated with clinical signs similar to those of canine monocystic ehrlichiosis (CME) developed revealing it a potentially zoonotic pathogen (Perez et al., 2006).
The genus Rhipicephalus (Acari: Ixodidae) includes several species with the R. sanguineus, the brown dog tick, group is frequently predominant in urban and rural areas of tropical and subtropical districts (Horak et al., 2013; Latrofa et al., 2014; Aktas, 2014; Malik et al., 2018) and most of them of veterinary and medical importance to dogs and humans (Dantas-Torres, 2008; Dantas-Torres et al., 2012; Latrofa et al., 2014). So far, the taxonomy of R. sanguineus comprises a complex issue with at least 4 taxa, particularly R. sanguineus sensu lato, have been recognized (Dantas-Torres et al., 2013a). Both A. platys and E. canis are evidently transmitted via the brown dog tick, R. sanguineus (Yabsley et al., 2008; Ramos et al., 2014).
In the past few decades, the PCR-based molecular detection of tick-borne pathogens in dogs has been increased. The present study aimed to detect the occurrence of Anaplasma platys and Ehrlichia canis in owned dogs in northern Egypt by the microscopic examination of Giemsa-stained blood films as well as molecularly by the use of conventional PCR in owned dogs’ blood.
MATERIALS AND METHODS
Study area and sampling
A total of 70 owned dogs (47 males and 23 females) were admitted, for a routine examination, treatment or vaccination, to private clinics in Alexandria, northern Egypt (coordinates: 31° 12′ 0″ N, 29° 55′ 0″ E). Dog breeds were German Shepherd (average weight 27-36 kg), Husky (average weight 17-25 kg), Golden Retriever (average weight 28-35 kg), Rottweiler (average weight 35-52 kg), Cane Corso (average weight 37-46 kg) and Mastiff (average weight 39-54 kg). Among the surveyed dogs, 42 animals were ticks-infested, 7 dogs were apparently ticks free but with a previous history of infestation and 21 dogs were not infested with ticks. According to the age, dogs are categorized into 3 sections; less than 2 years (40 dogs), 2-5 years (24 dogs) and more than 5 years (6 dogs). The whole blood (n=70) from the cephalic vein was taken by the use of 5 ml-syringe and added to tubes containing ethylene diamine tetraacetic acid (EDTA).
Microscopic examination
Thin blood smears were fixed in methanol (99.5%) for 5 min and stained for 30 min in Giemsa solution diluted with 5% buffer. Slides were carefully examined for the occurrence of members of Anaplasmataceae. The smears were recorded as negative if no pathogens were detected in more than 50 oil-immersion microscopic fields (El-Dakhly et al., 2018).
DNA extraction and PCR amplification
The remaining blood was used for PCR. DNA was extracted from 200 μl of EDTA-anticoagulated blood samples from dogs using the purification kit QIAamp DNA blood mini kit® (QIAGEN GmbH, Hilden, Germany). PCR amplification for the detection Anaplasma spp./ Ehrlichia spp. and Babesia spp./ Hepatozoon spp. was conducted using 25 µl-volume reactions: 12.5 master mix, 1 µl forward primer, 1 µl reverse primer, 7.5 µl DNA and RNA free water and 3 µl DNA from each sample. The used primers and cycling conditions were listed in Table 1.
Table 1: Primers and cycling conditions of the tested pathogens in examined dogs.
| Parasite | Primer | Target gene | Product size (bp) | Cyclic conditions | References |
|
Anaplasma spp./ Ehrlichia spp.
|
EHR16SD: 5′-GGTACCYACAGAAGAAGTCC- 3 ′ EHR16SR: 5′-TAGCACTCATCGTTTACA GC-3 |
16S rRNA | 345 | 95°C-120 sec, 94°C-60 sec, 54°C-30 sec (x40), 72°C-30 sec, 72°C- 300 sec | |
|
Babesia spp./ Hepatozoon spp. |
B18S-F 5′-TGG TTG ATC CTG CCA GTA-3′ B18S-R 5′-CTT CTC CTT CCT TTA AGT TGA-3′ |
18S rRNA | 1665 | 94 °C- 30 sec,94 °C- 30 sec,56 °C- 2min (x30),72 °C- 2 min,72 °C- 5 min. |
RESULTS AND DISCUSSION
The clinical examination of dogs revealed that 42 (60.0%) out of 70 were infested with ticks, Rhipicephalus sanguineus. Among the tick-infested dogs, 27.14% (19/70) had loss of appetite, loss of body weight and lethargy. The remaining dogs (40.0%; 28/70) were submitted for the routine examination and vaccination.
The microscopic examination of 70 Giemsa-stained blood smears (in less than 50 microscopic fields) revealed 6 (8.57%) dogs were infected with Anaplasma/Ehrlichia spp. The intensity of infection ranged from 1 to 3 protozoan/field. Among those, 5.71% (4/70) were only positive for Anaplasma platys. The pathogen was recognized as inclusion bodies in the blood platelets of infected dogs (Figure 1). Moreover, Ehrlichia canis was found in 2 (2.86%) dogs. It appeared as multiple rounded-shaped and relatively large-sized morulae inside macrophages (Figure 2). Dogs infected with both pathogens were infested with the associated brown dog tick, Rhipicephalus sanguineus, with the intensity of infestation ranged from 1-16 ticks (Figure 3). Based on the microscopic examination, no mixed infections were detected.
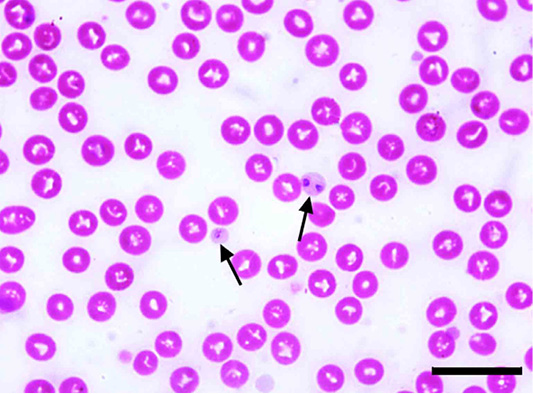
Figure 1: Anaplasma platys inclusion bodies in blood platelets (arrows) of Giemsa-stained blood smear of an infected dog. Scale bar= 20 um.
Surprisingly, neither Babesia nor Hepatozoon species could be detected in the examined samples. The examination of more than 50 fields was almost doubtful with a great tendency for all samples to be Babesia/Hepatozoon free. Therefore, a specific primer for both was used to absolutely exclude the absence of those pathogens.
Concerning the age, it has been found that dogs aged less than 2 years were highly infected, while elderly dogs were the lowest (Table 2). The current study revealed that male dogs were highly infected than females (Table 3).
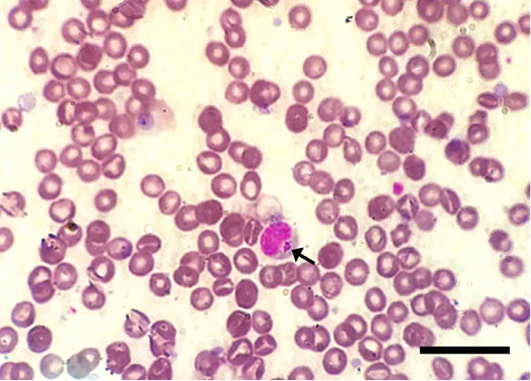
Figure 2: Ehrlichia canis inside a macrophage in Giemsa-stained blood smear of an infected dog. Note the multiple, relatively large-sized and round-shaped morulae intracellularly (arrows). Scale bar= 20 um.
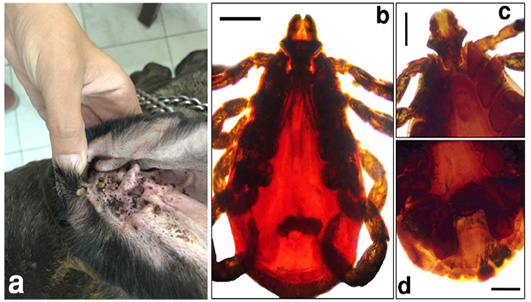
Figure 3: The adult brown tick, Rhipicephalus sanguineus, infesting the surveyed dogs. a) A dog ear infested with the tick vector. b) Doral view of the adult tick. c) Anteroventral view of the adult tick. d) Posterventral view of the adult tick. Scale bar= 0.5 mm.
Table 2: The infection of Anaplasma/Ehrlichia species relative to the age of surveyed dogs.
|
Anaplasma/Ehrlichia infected dogs (%) |
Examined dogs | Age |
| 9 (12.86%) | 40 | < 2 years |
| 3 (4.28%) | 24 | 2-5 years |
| 2 (2.86%) | 6 | >5 years |
| 14 (20.0%) | 70 | Total |
Table 3: The infection of Anaplasma/Ehrlichia species relative to the gender of surveyed dogs.
|
Anaplasma/Ehrlichia infected dogs (%) |
Examined dogs | Gender |
| 9 (12.86%) | 47 | Male |
| 5 (7.14%) | 23 | Female |
| 14 (20.0%) | 70 | Total |
The molecular investigation of the surveyed samples showed that 20.0% (14/70) of dogs were positive for Anaplasma/ Ehrlichia species, using a common primer for both pathogens, with a specific amplicon size of 345 bp (Figure 4). Babesia/Hepatozoon species could not be detected among the examined samples. Expectedly, the use of PCR-based molecular tool was more accurate than the conventional direct microscopy.

Figure 4: PCR amplification of DNA extracted from blood of surveyed dogs targeting the 16S rRNA gene using a primer specific for Anaplasma/Ehrlichia spp. Lane M: a ladder of 100 bp. Lanes 1,2 showed control negative and control positive respectively. Lanes 3–12 showed 10 blood samples positive for Anaplasma/Ehrlichia spp. with a product of an amplicon size 345 bp.
The occurrence of canine vector-borne pathogens is a global concern for both pets’ clinics and public health (Maggi et al., 2013; Ribeiro et al., 2017). The infection with Anaplasmataceae often based on the pathogen species, immune status of the host as well as the co-infections with other pathogens (Dahmani et al., 2019). The current work revealed that A. platys and E. canis were found in blood of examined dogs by the use of both microscopic examination and PCR, in Alexandria, northern Egypt. More or less similarly, Botros et al. (1995) examined both military kenneled and private owned dogs and they revealed that the overall ehrlichiosis was 33.0% using florescence antibody test. A recent study in Giza, Egypt showed that, based on PCR, percentages were 1.32% and 1.98% for both A. platys and E. canis, respectively. The discrepancy in percentages of infection might be due to the topography of both surveyed provinces as well as it might be attributed to the source of extracted DNA used in PCR (DNA is extracted from ticks in the later study and from blood in the current study) (Nasr et al., 2020). Meanwhile, Selim et al. (2021) recovered that the prevalence of E. canis using PCR-based molecular identification was 9.70%. Moreover, another report in Egypt demonstrated clinical signs of canine ehrlichiosis in 85 dogs without the detection of the pathogen by the microscopic examination in Giemsa-stained smears (Salem et el., 2014). Furthermore, Ghafar and Amer (2012) found that the prevalence of A. phagocytophilum in ticks associated with dogs was 13.7% based on PCR targeting 16S rRNA gene.
In other Arabian countries, like Qatar, based on PCR findings, infection rates for Anaplasma platys and Ehrlichia canis were 1.6% and 3.1%, respectively. The lower percentage than the current investigation, 20.0%, might be referred to more veterinary care. Zaid et al. (2019) recorded that the infection rate of A. platys and E. canis in dogs blood samples in 9 districts of Palestine was 10% and 1.5%, respectively.
Closely related to our findings, Dahmani et al. (2019) reported infection rates of 15.6% and 18.8% for A. platys and E. canis, respectively using molecular investigation in Keur Momar Sarr area, Senegal. The later is an African country probably assuming more or less similar climate changes and topographical nature providing the existence of the same tick vector, Rhipicephalus sanguineus. In Nigeria, Adamu et al. (2014) recovered that either A. platys or E. canis was found in 7.0% of the surveyed dogs using PCR and RLB (Reverse Line Blot) assays. In Harare, Zimbabwe, Dhliwayo et al. (2019) detected that the overall prevalence of canine ehrlichiosis was 75.2% using ELISA. The higher infection rate might be attributed to the possible false results which could be achieved.
Out of the African continent, variable prevalences of anaplasmosis/ehrlichiosis were obtained. Among them, In Grenada, Yabsley et al. (2008) noted that the percentages of A. platys and E. canis infections were 19.2% and 24.7%, respectively. Kelly et al. (2013) reported that prevalences of A. platys and E. canis using 16S rRNA-based PCR in dogs St. Kitts in the Caribbean were 11.0% and 27.0%, respectively. In Brazil, Tanikawa et al. (2013) found that the infection rate with E. canis was 69.4% and 3.7% based on serological assay and RT-PCR, respectively. Ramos et al. (2014) detected that 18 (52.9%) dogs were positive for A. platys in southern Italy. Almazan et al. (2016) revealed that 31.0% and 10.0% of examined dogs were positive to A. platys and E. canis, respectively using 16S rRNA amplification-based PCR in north-central Mexico. Ybañez et al. (2018) detected that the prevalence of Anaplasma/Ehrlichia infection in dogs using peripheral blood smear examination and PCR was 10.0% in Cebu, Philippines. Ebani (2019) found that 16.18% of samples were positive for E. canis and 3.31% for A. phagocytophilum using an indirect immunofluorescent assay in Italy. Amazingly, Zheng et al. (2017) reported neither Anaplasma platys nor Ehrlichia canis infection in surveyed dogs in 10 provinces of China. The current study reported that the use of advanced tools like 16S RNA-dependant PCR is more accurate than Giemsa-stained direct microscopy for detecting either A. platys or E. canis as a result of artifacts of the staining procedure as well as the misdiagnosis of the pathogen morulae intracellularly (Kelly et al., 2013; Salem et al., 2014; Almazan et al., 2016).
Currently, the percentage of anaplasmosis/ehrlichiosis in dogs aged less than one year were the highest, while dogs of more than 5 years were the lowest with male dogs were highly infected than females. Authors suggested that this issue might be due to a less veterinary care in the beginning of life together with the abundance of the tick vector, R. sanguineus, in the locality where dogs are sampled (Botros et al., 1995). Unlike these findings, Tanikawa et al. (2013) and Selim et al. (2020) reported that elderly dogs were more susceptible to the infections with both A. platys and E. canis. They referred this finding to the fact that aged dogs are more exposed to the infection together with the persistence of the tick vector along their life. Meanwhile, Ribeiro et al. (2017), Malik et al. (2018) and Dhliwayo et al. (2019) found that age, breed and sex have no effect on anaplasmosis/ehrlichiosis. Interestingly, Díaz-Sánchez et al. (2020) reported that the existence of ticks is not correlated with the severity of the infection.
The brown dog tick, R. sanguineus, is the main vector for the transmission of Anaplasma platys and Ehrlichia canis in tropical and subtropical areas. In Egypt, the detection of those pathogens in dogs and their associated ticks in various localities of the Mediterranean area is potentially correlated to the climate changes and ecosystem suggesting the continuation of the dog-tick life cycle (Pennisi et al., 2012; Dantas-Torres et al., 2013b; Latrofa et al., 2014; Almazán et al., 2016). It is worthy to mention that ticks seemed to be pathogens-free due to being that dogs are apparently healthy, as in previously treated dogs, therefore, the circulating antigens could not be detected (Almazán et al., 2016).
In the authors opinion, the overall discrepancies in the prevalence’s of anaplasmosis/ehrlichiosis might be referred to a variety of factors; the lack of veterinary care and awareness of people towards dog populations particularly in poorly developed districts, climatic changes and topographical characters in several localities in such a way to allow the wide spread and extent of tick infestation in dogs, diagnostic tools used as well as the close contact between dogs and humans providing the potential role of those pathogens as zoonotic vector-borne pathogens (Galay et al., 2018). Due to the highly possible morbidity and mortality, biologists and veterinarians have to be exclusively alert to provide a well veterinarian care as well as the elimination of associated ticks that will reduce the spread of those tick-borne pathogens. Thus, the use of both direct microscopy and PCR-based molecular tools are highly recommended.
ACKNOWLEDGMENTS
Authors are thankful to veterinarians in clinics who facilitated the sampling in this work.
Novelty Statement
Authors declare that the manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
AUTHOR’S CONTRIBUTION
Khaled Mohamed El-Dakhly examined dog samples, collected raw data, identified pathogens and wrote the manuscript. Waleed M. Arafa examined samples, stained blood films and did PCR, Lilian N Mahrous contributed in editing, Amany S. Aboshinaf examined blood samples and did PCR. Magdy M. Tawfik collected samples, stained blood smears, did PCR.
Compliance with ethical standards
Dog blood samples were collected in clinics according to well practice by veterinarians and according to the Egyptian regulations with the agreement of the owners.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES





